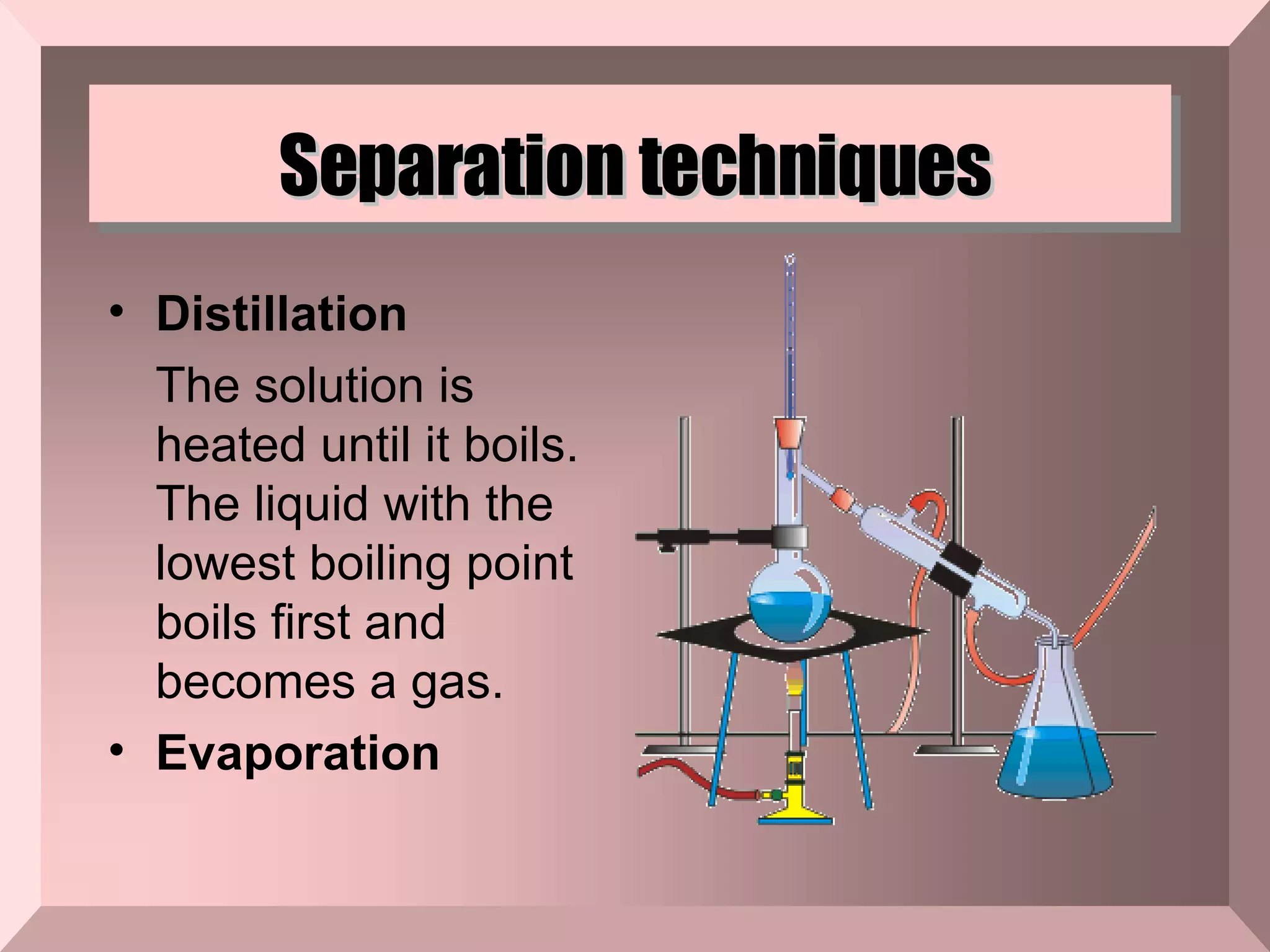
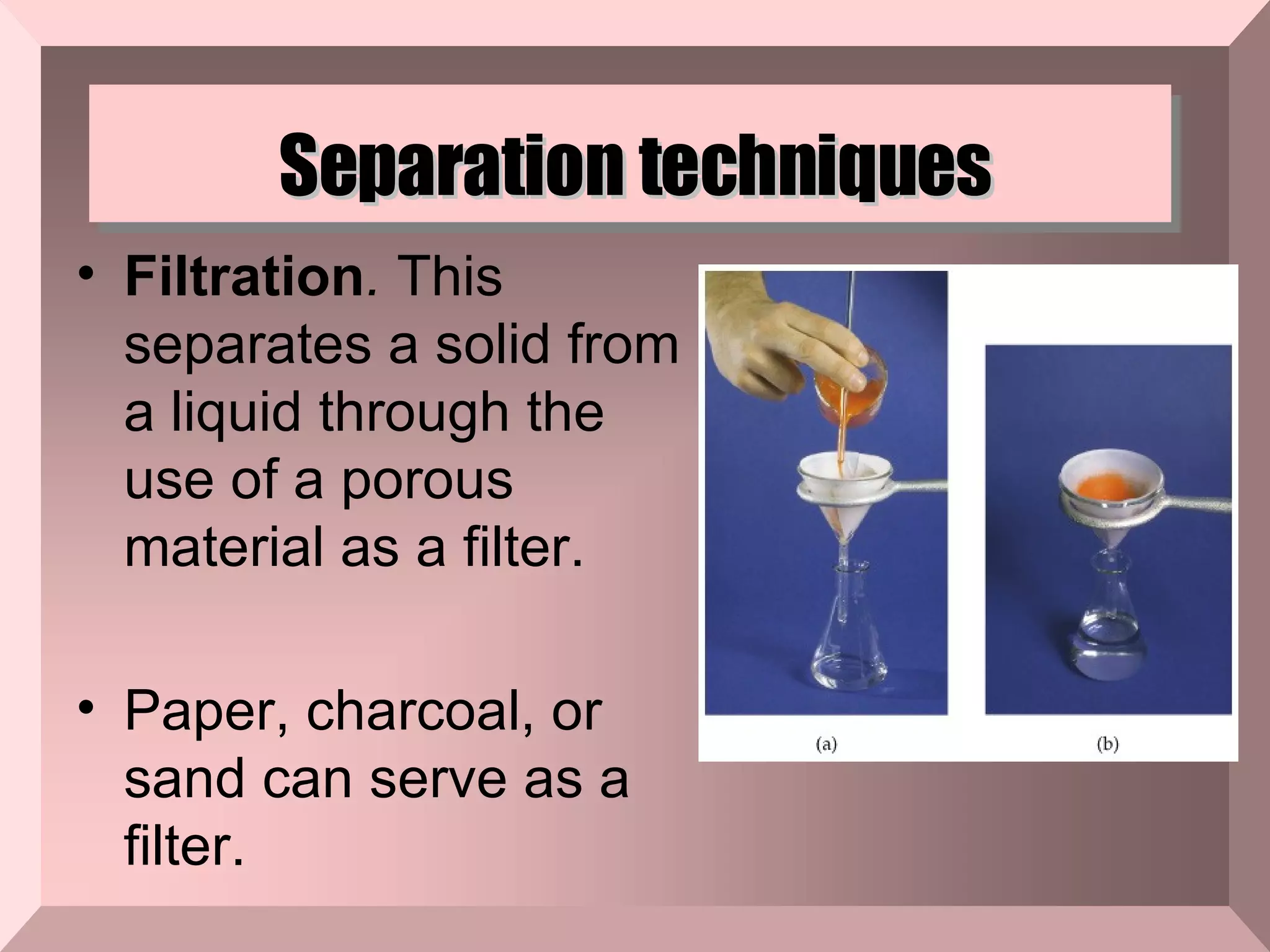
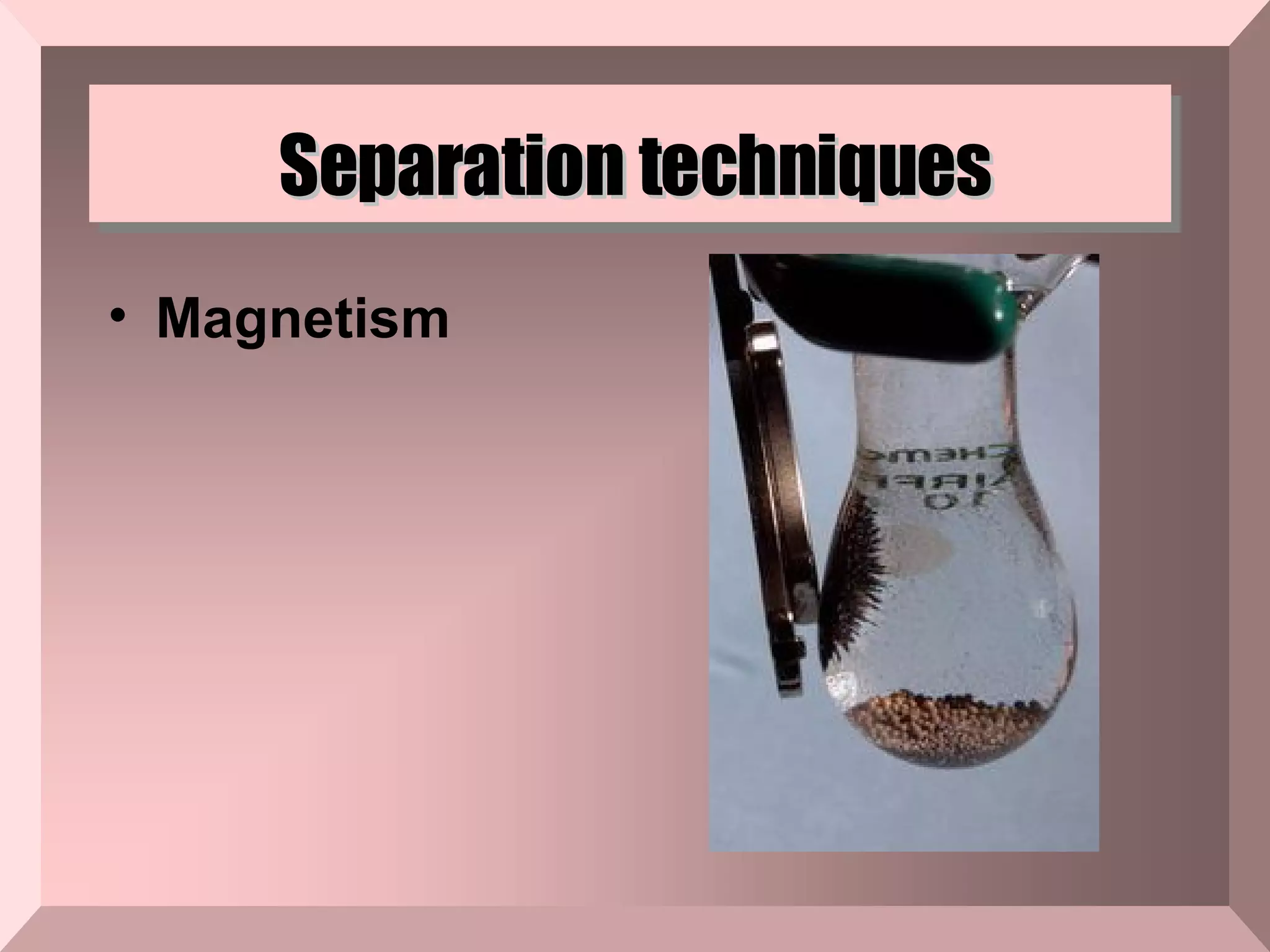
The document describes different types of mixtures and separation techniques. It defines a mixture as two or more substances combined without a chemical reaction that maintain their individual properties. Mixtures are either heterogeneous, with components visually distinguishable, or homogeneous. Common separation techniques include distillation, filtration, chromatography, and others that separate mixtures based on differences in their boiling points, ability to pass through filters, or interactions with magnetic or other fields.