Embed presentation
Downloaded 69 times

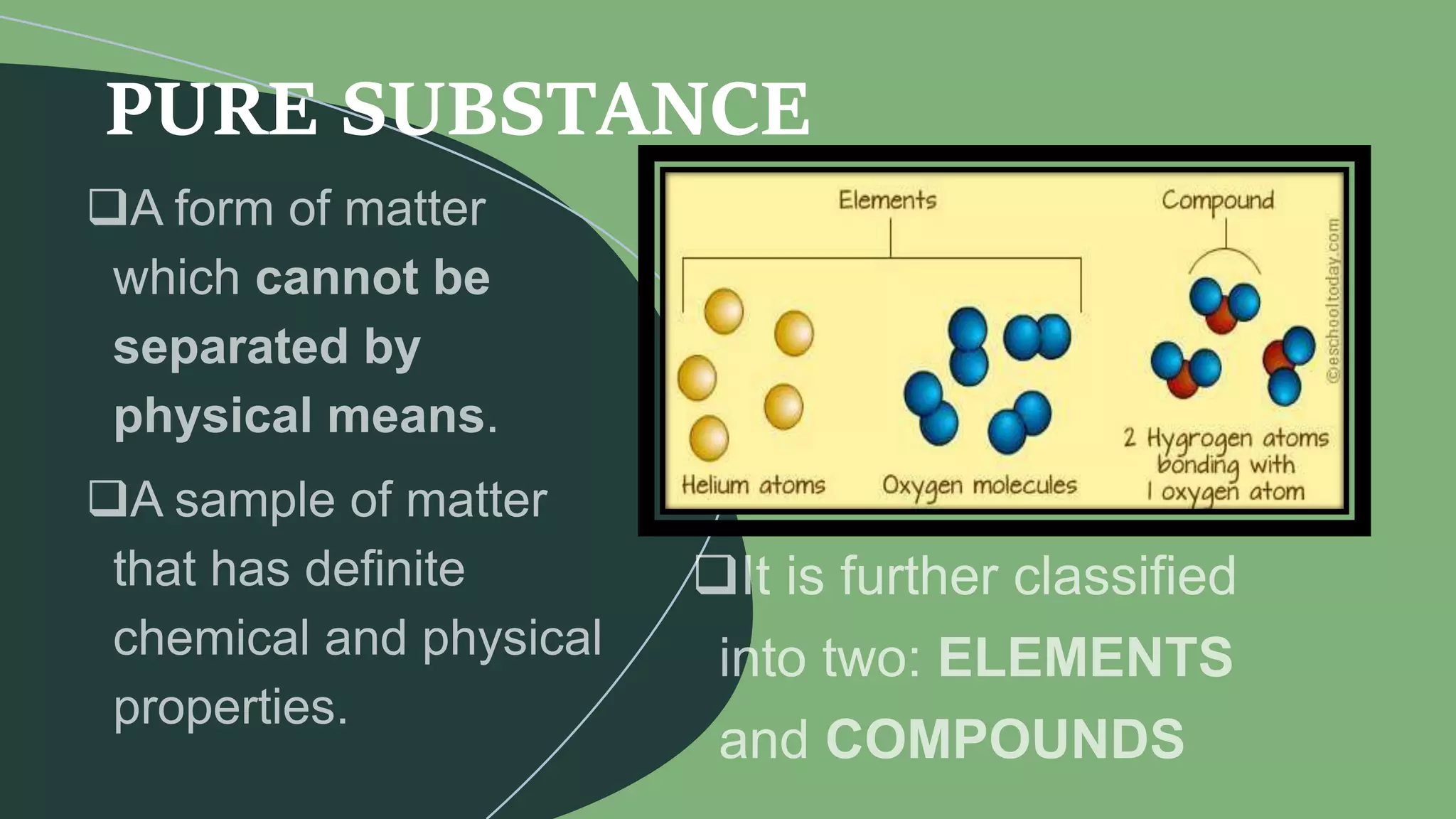
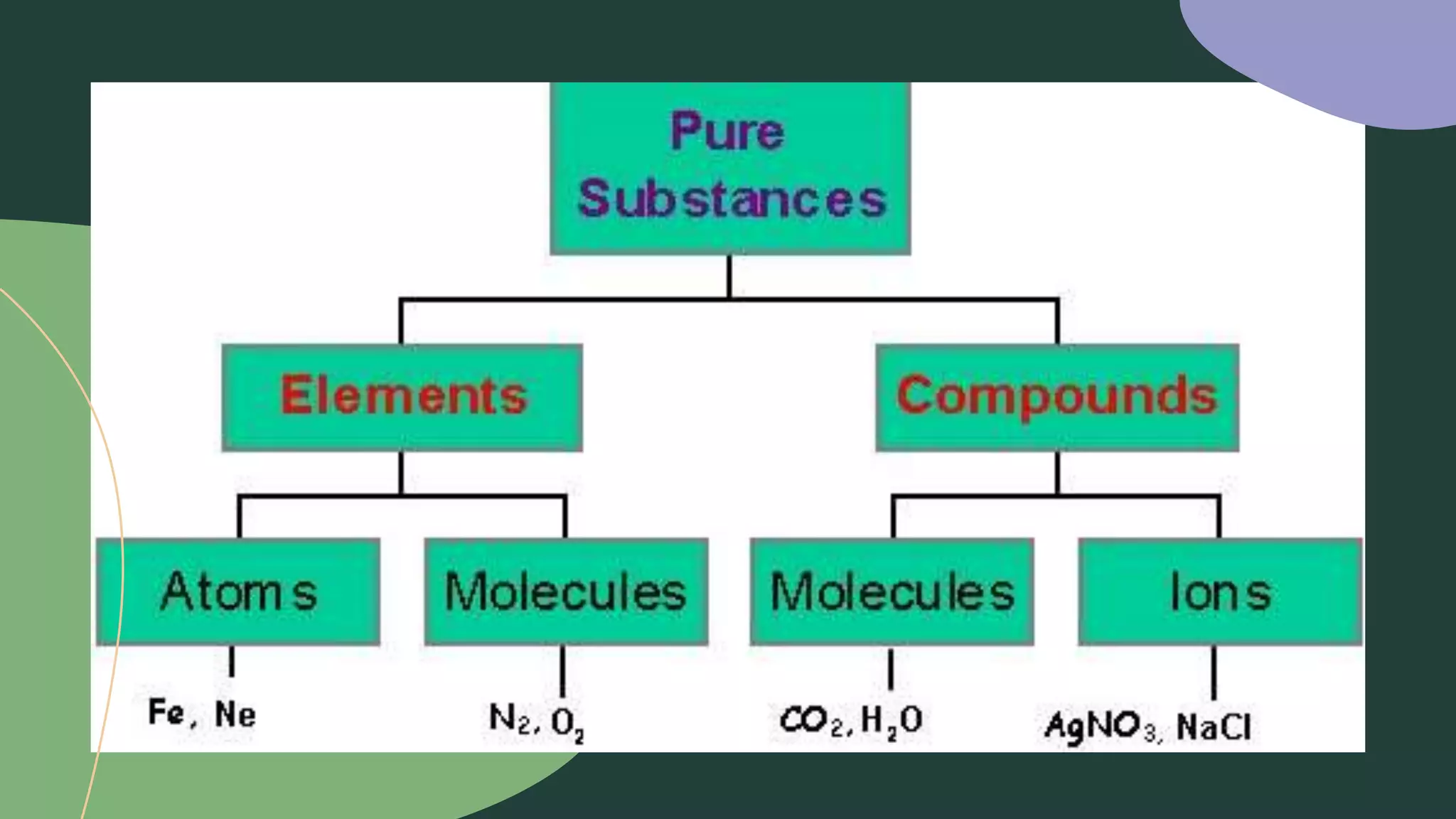
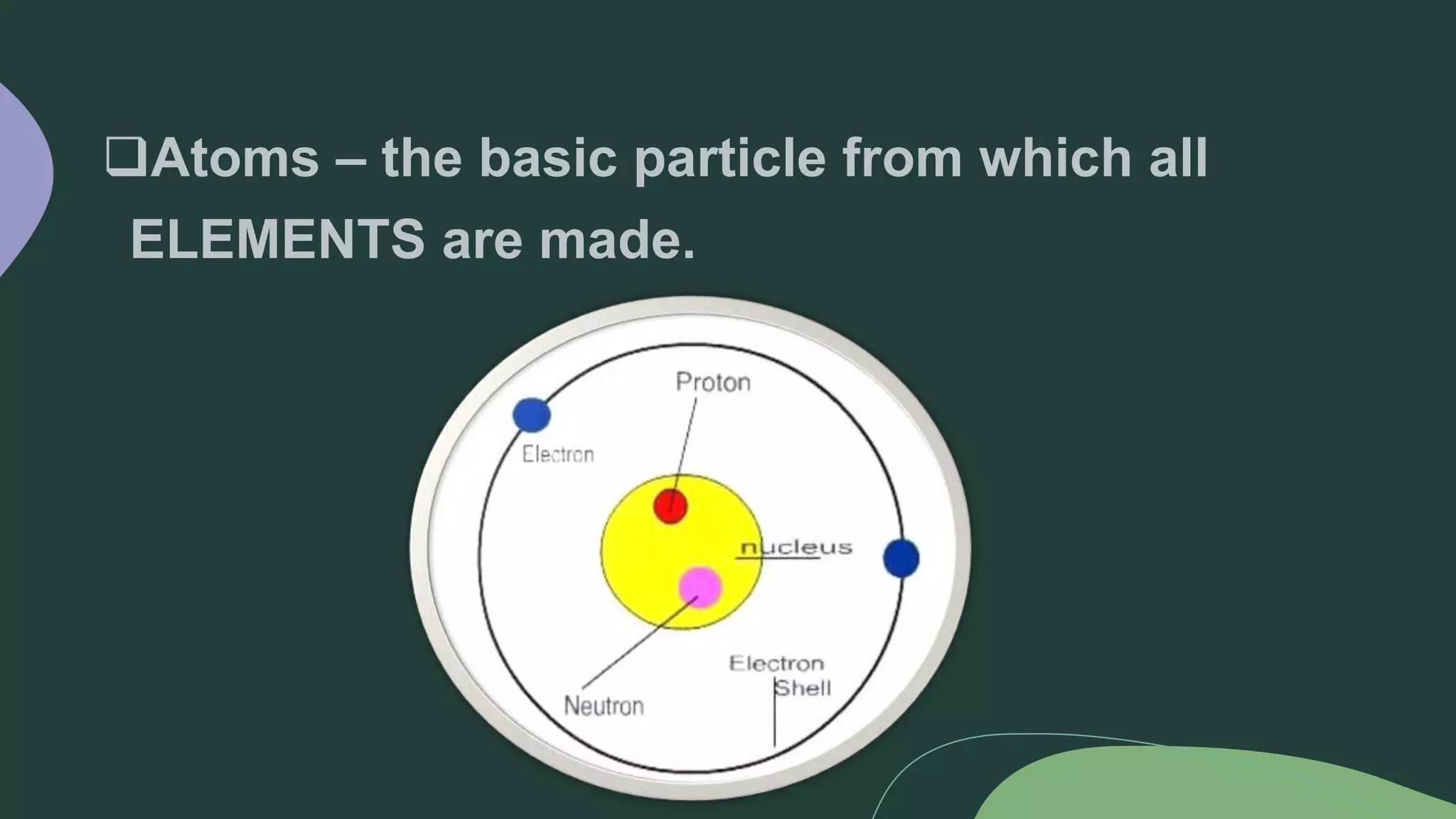

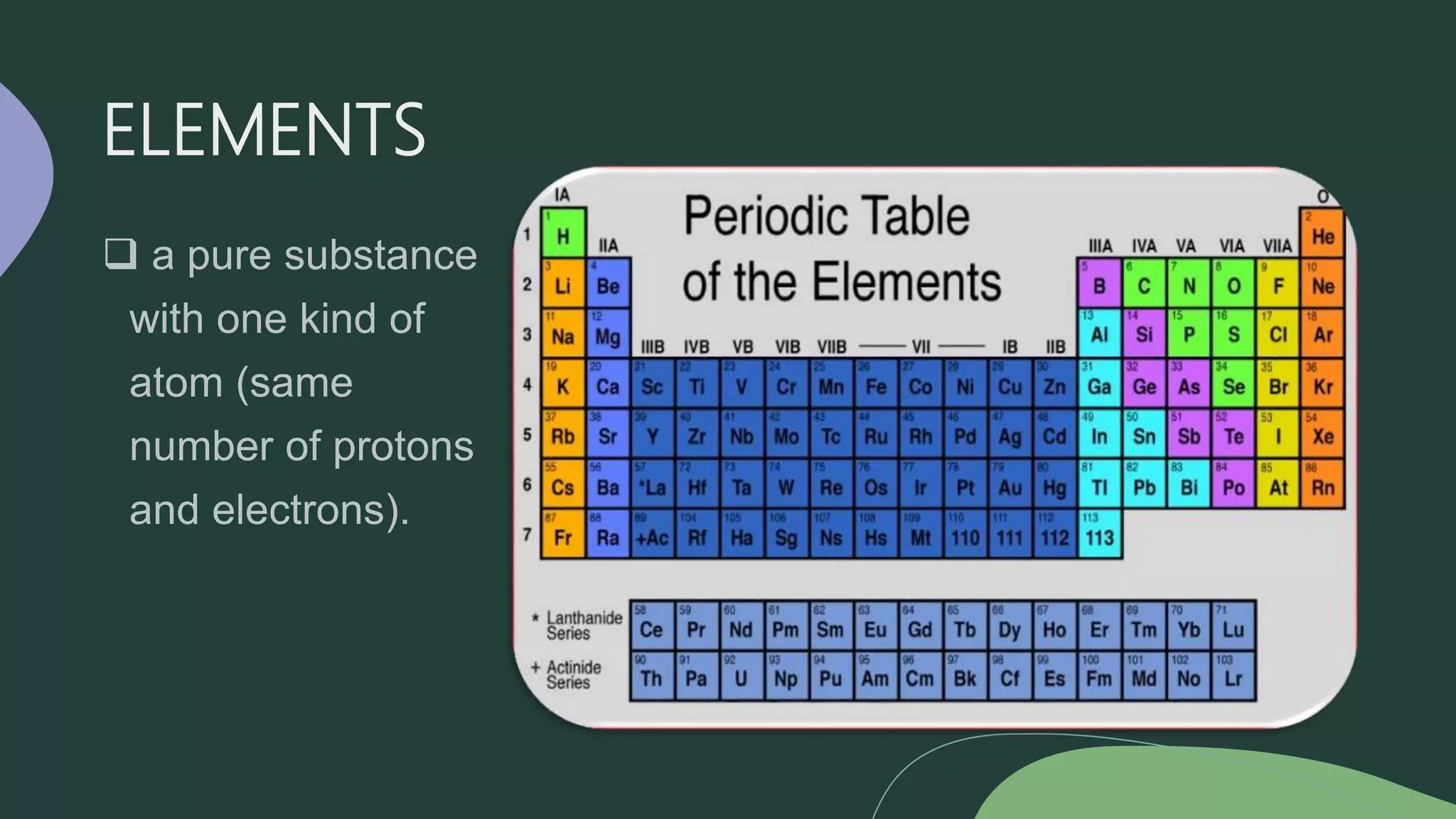











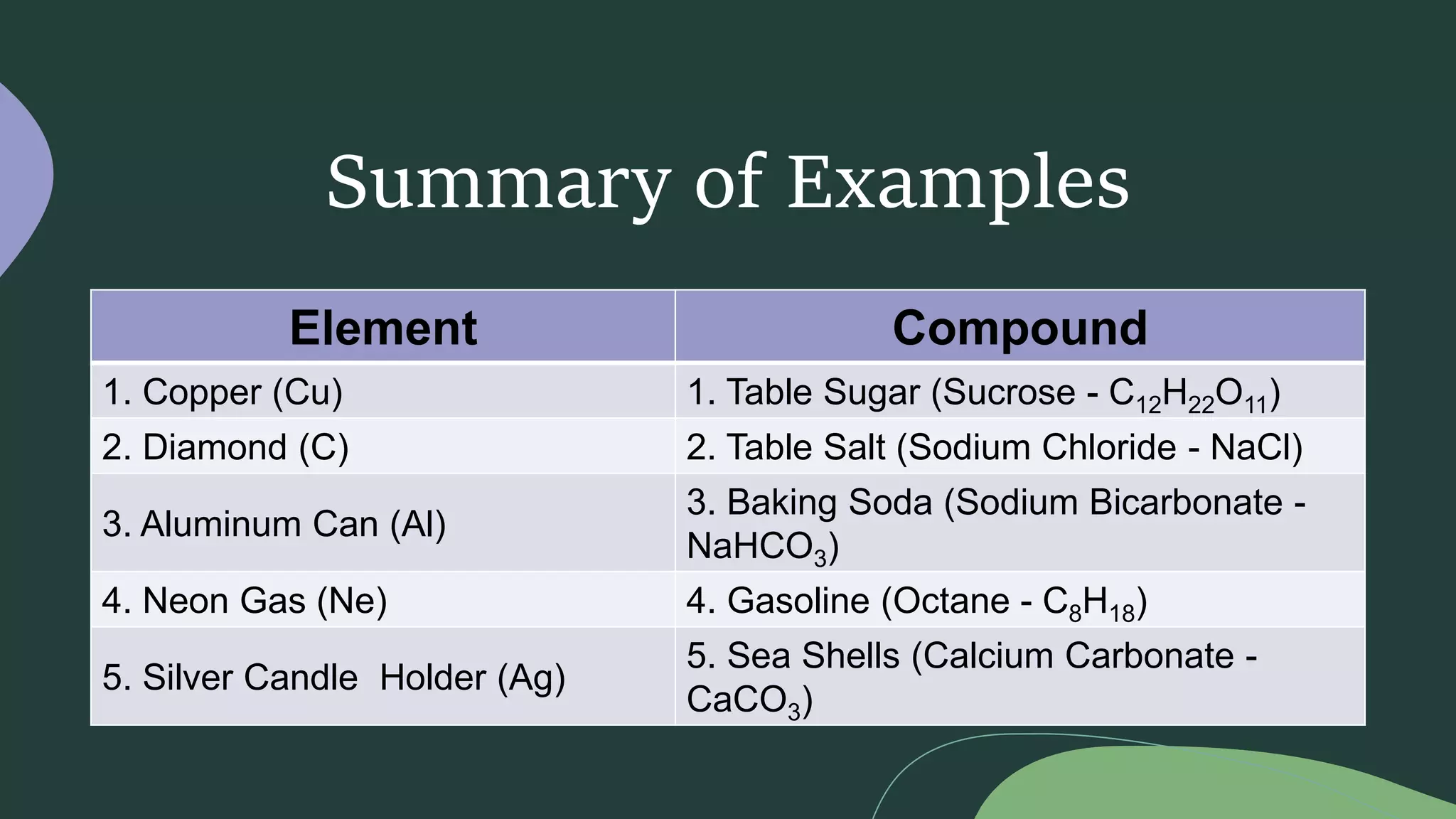

This document discusses the difference between elements and compounds. Elements contain only one type of atom and cannot be broken down further, while compounds contain two or more types of atoms and can only be separated through chemical means. Examples are provided of common elements like copper, carbon, and neon as well as compounds including table salt, baking soda, and gasoline to illustrate the difference between pure substances that are elements versus those that are compounds consisting of multiple elements bonded together.



















