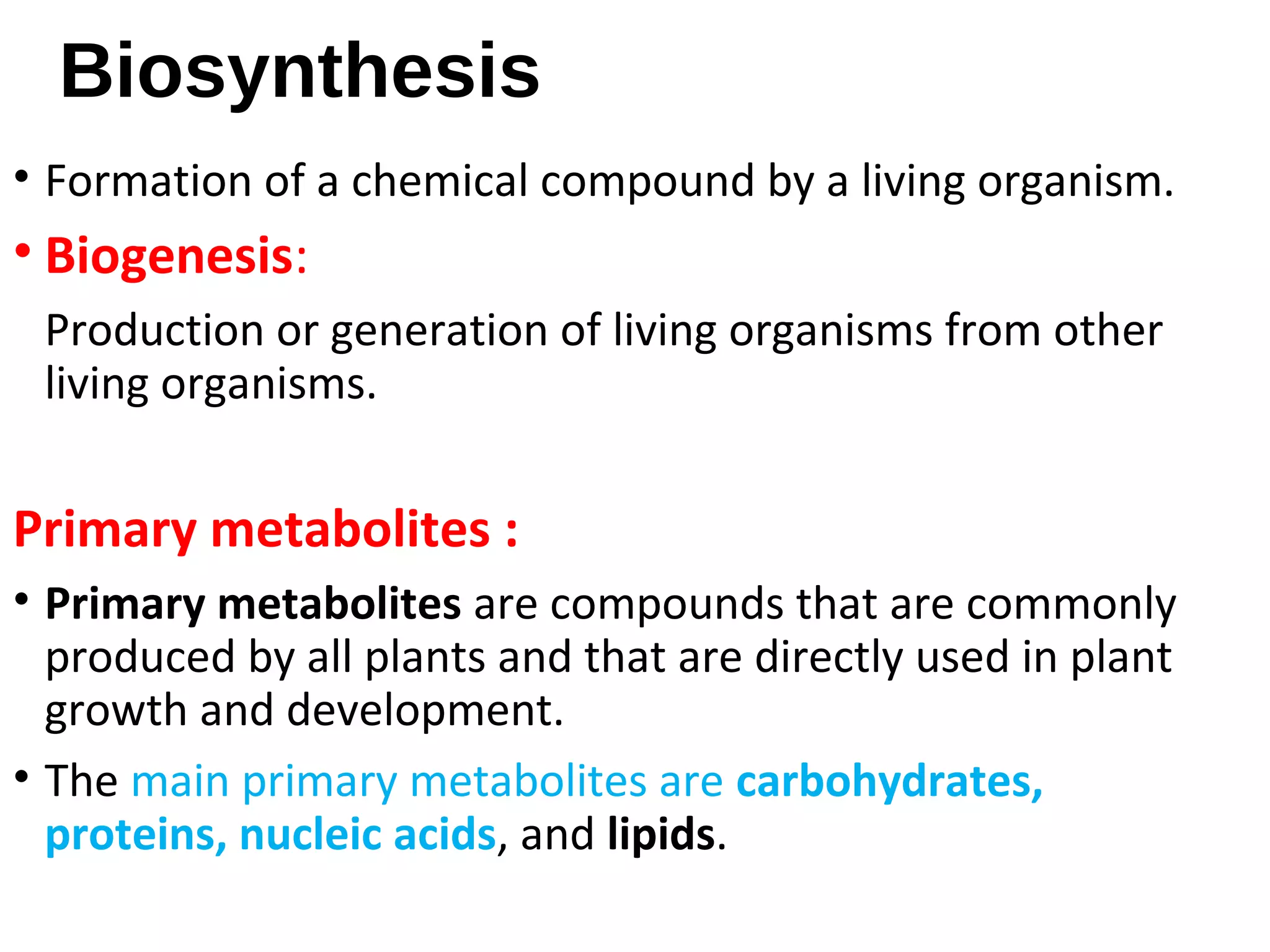
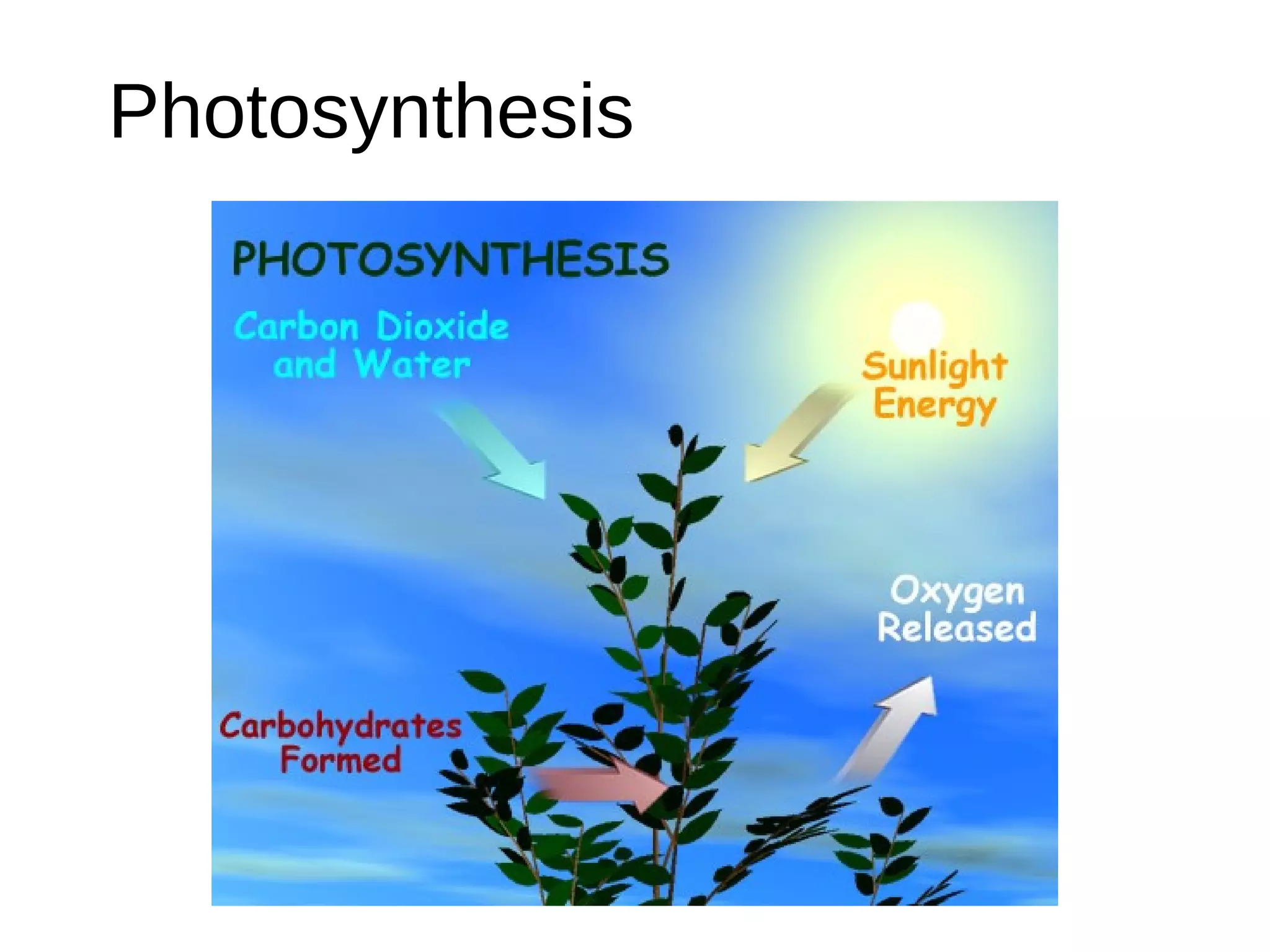
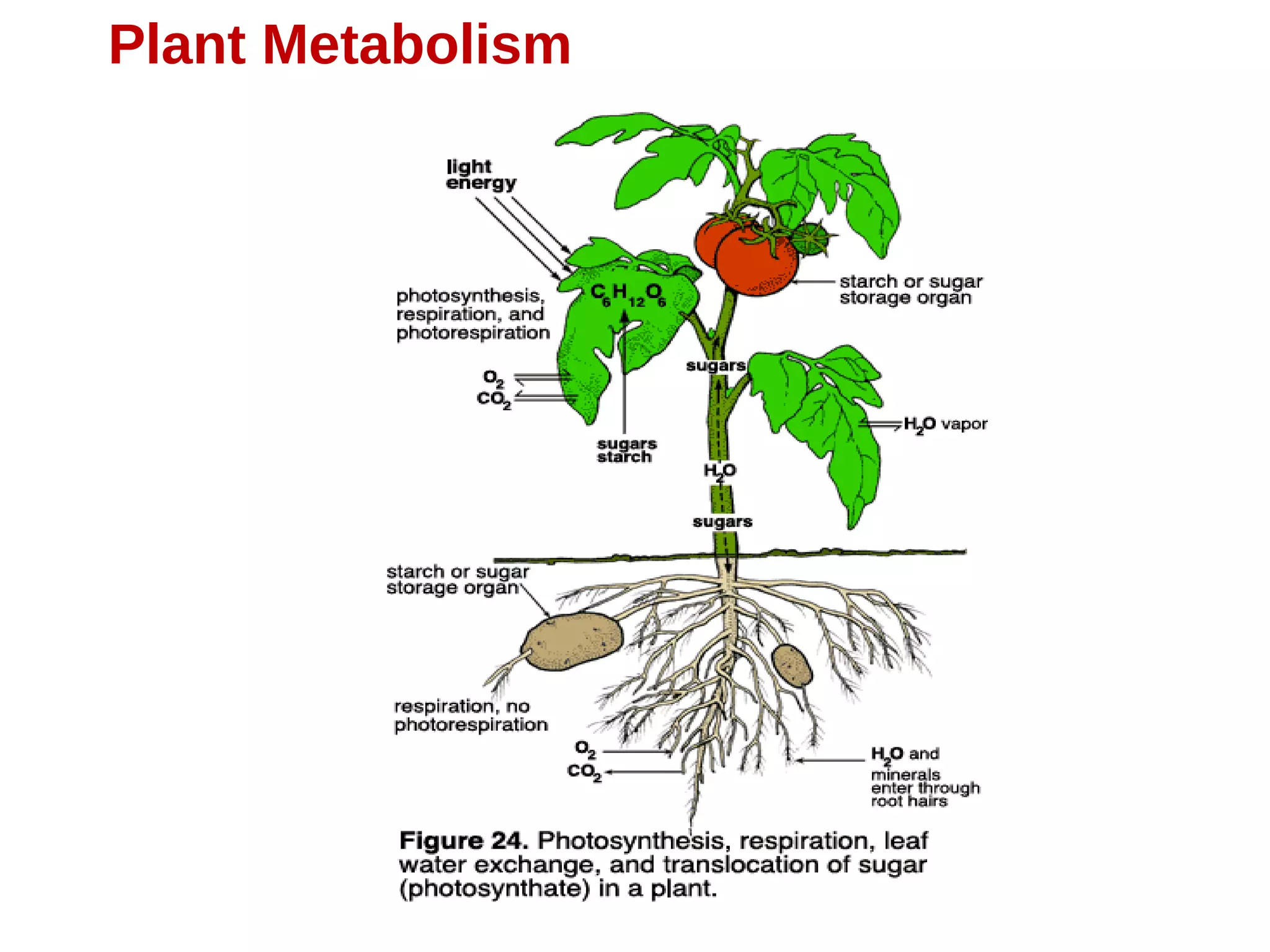
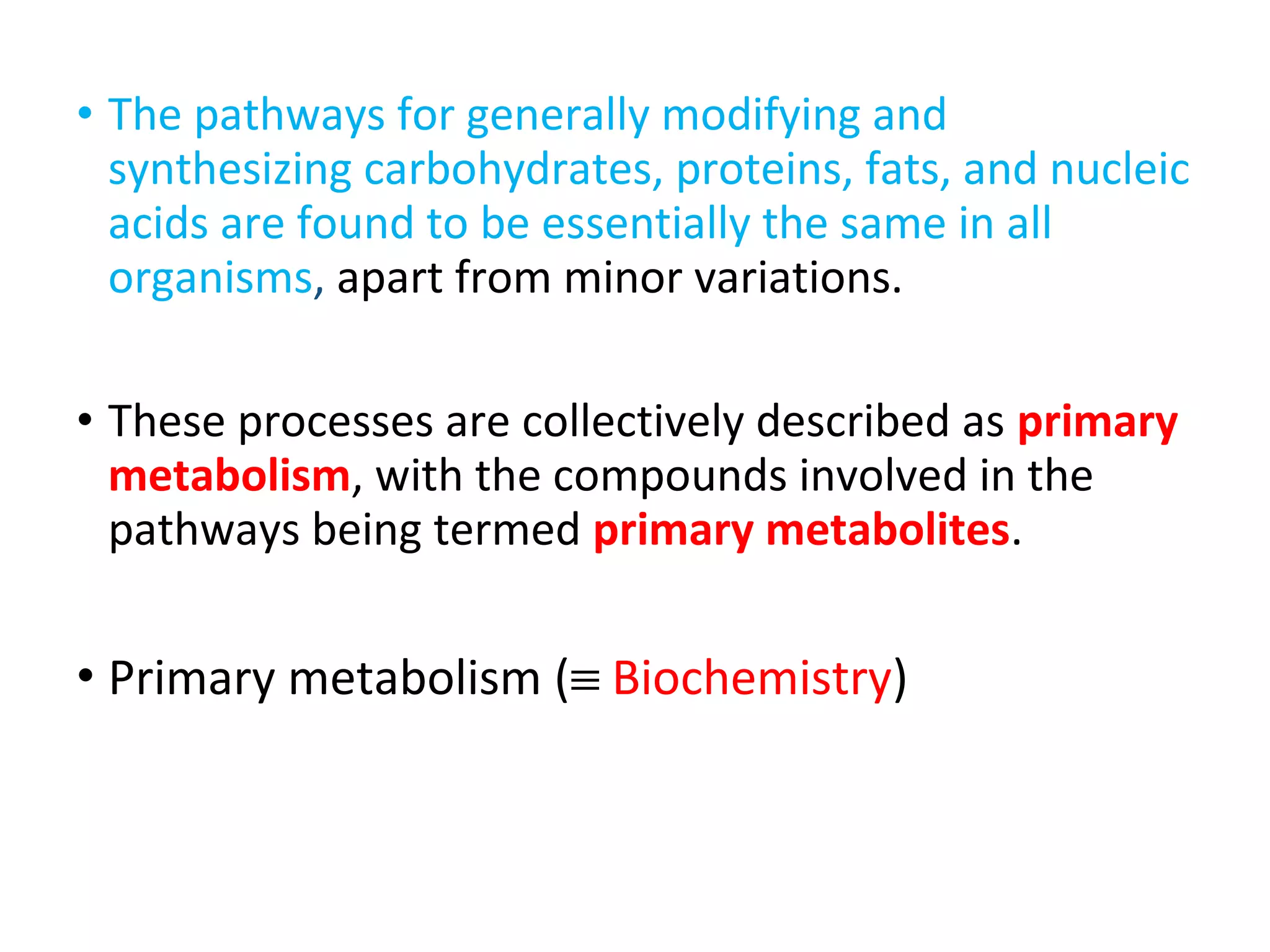
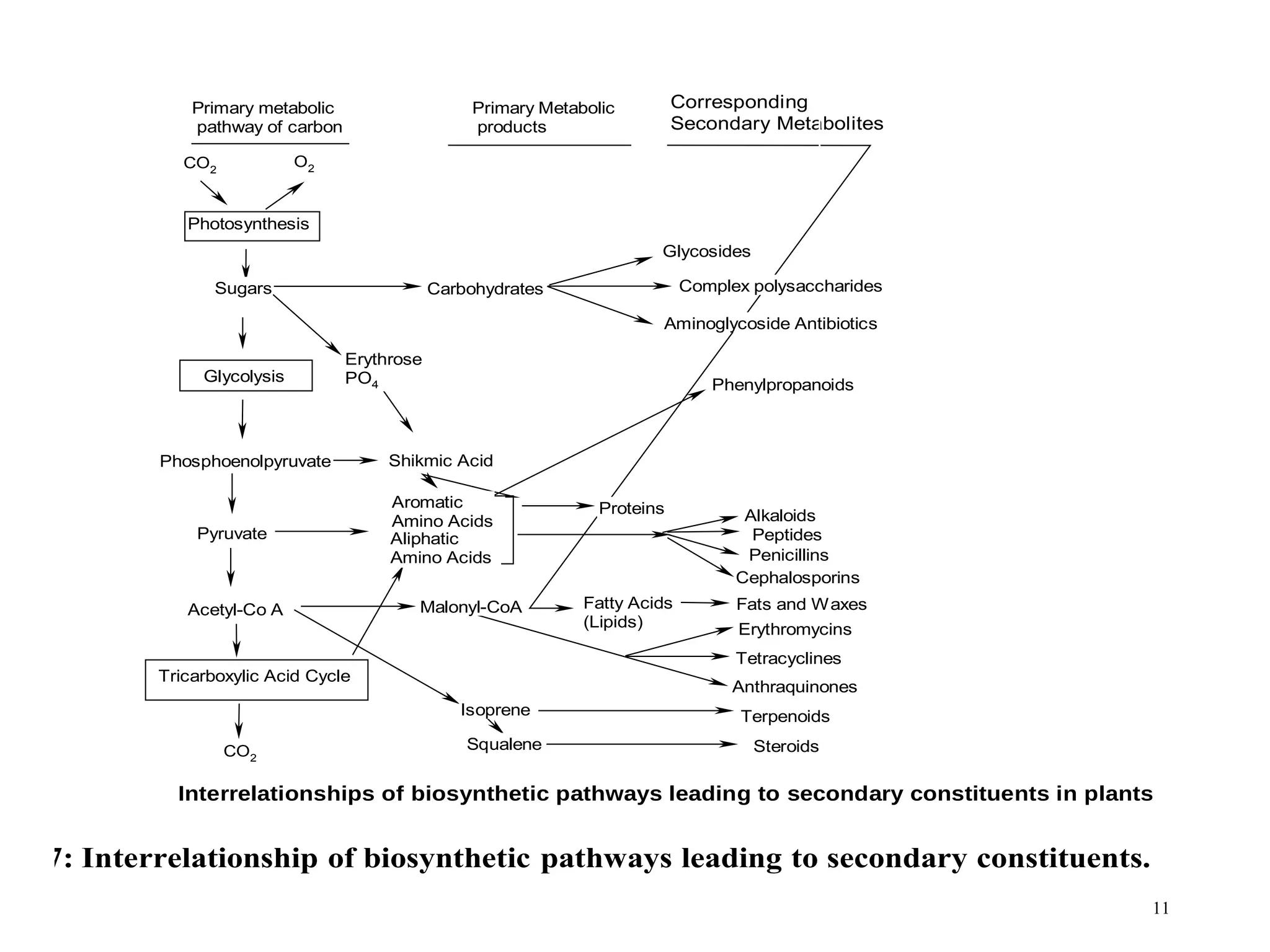
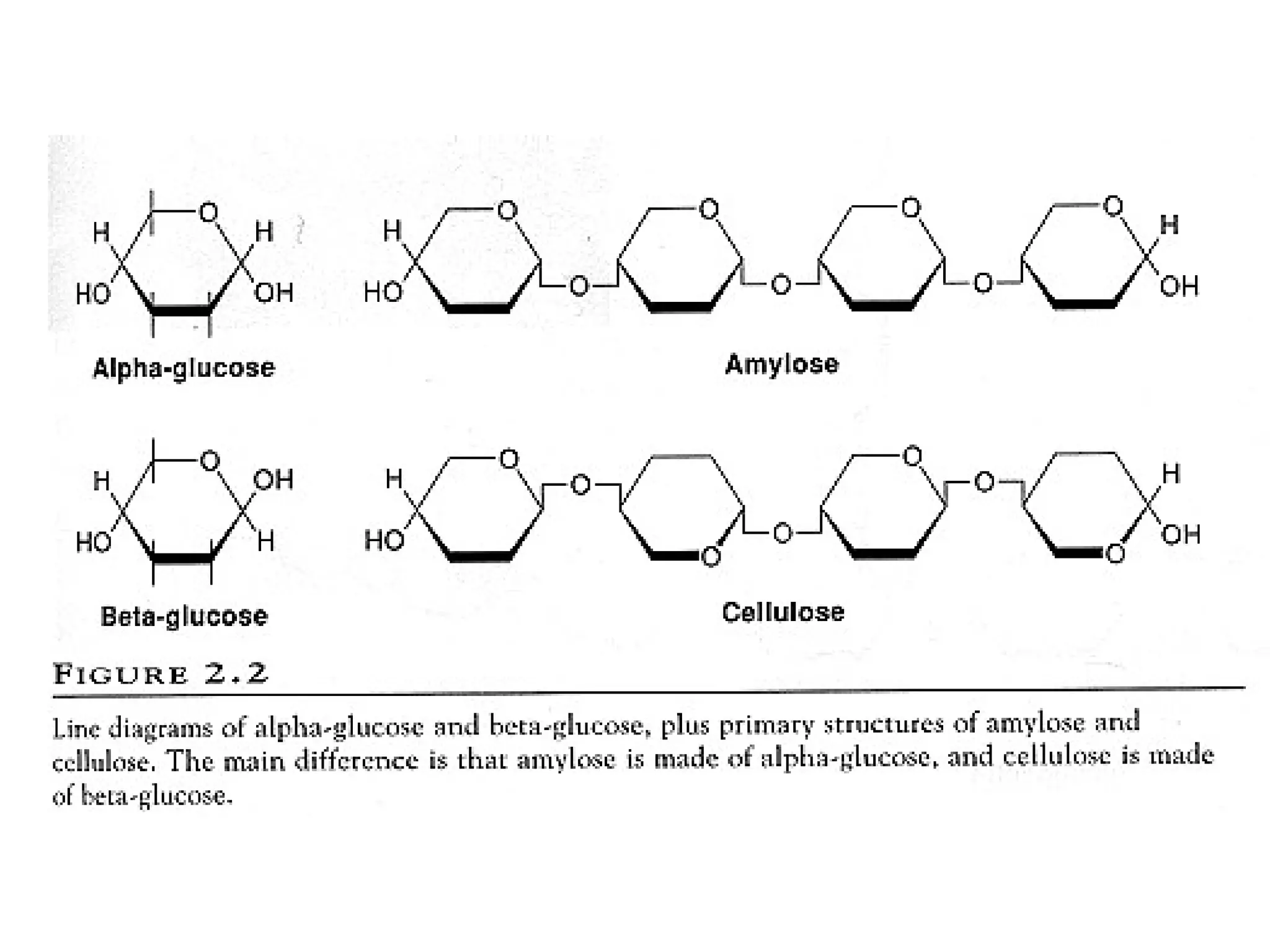
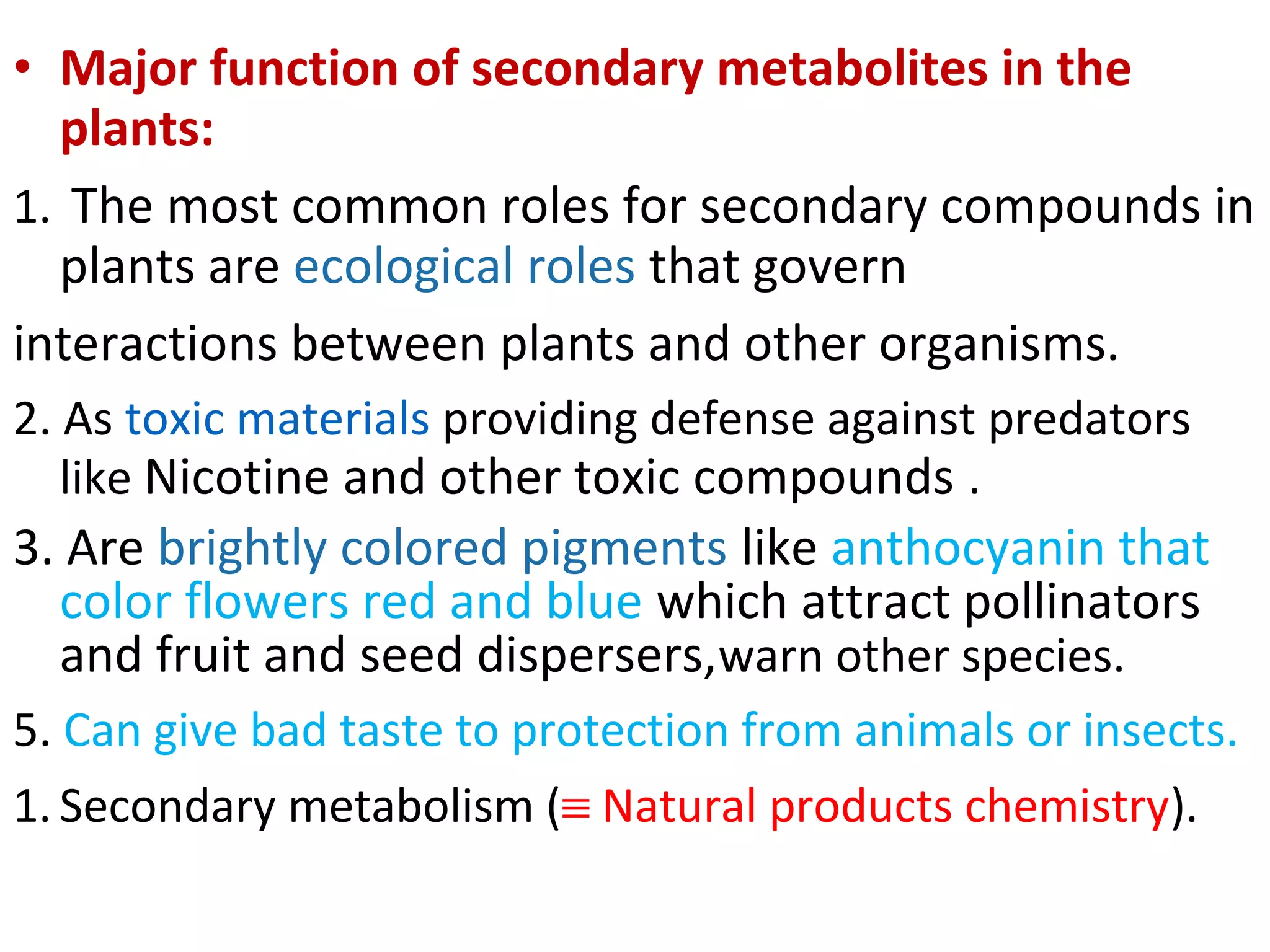
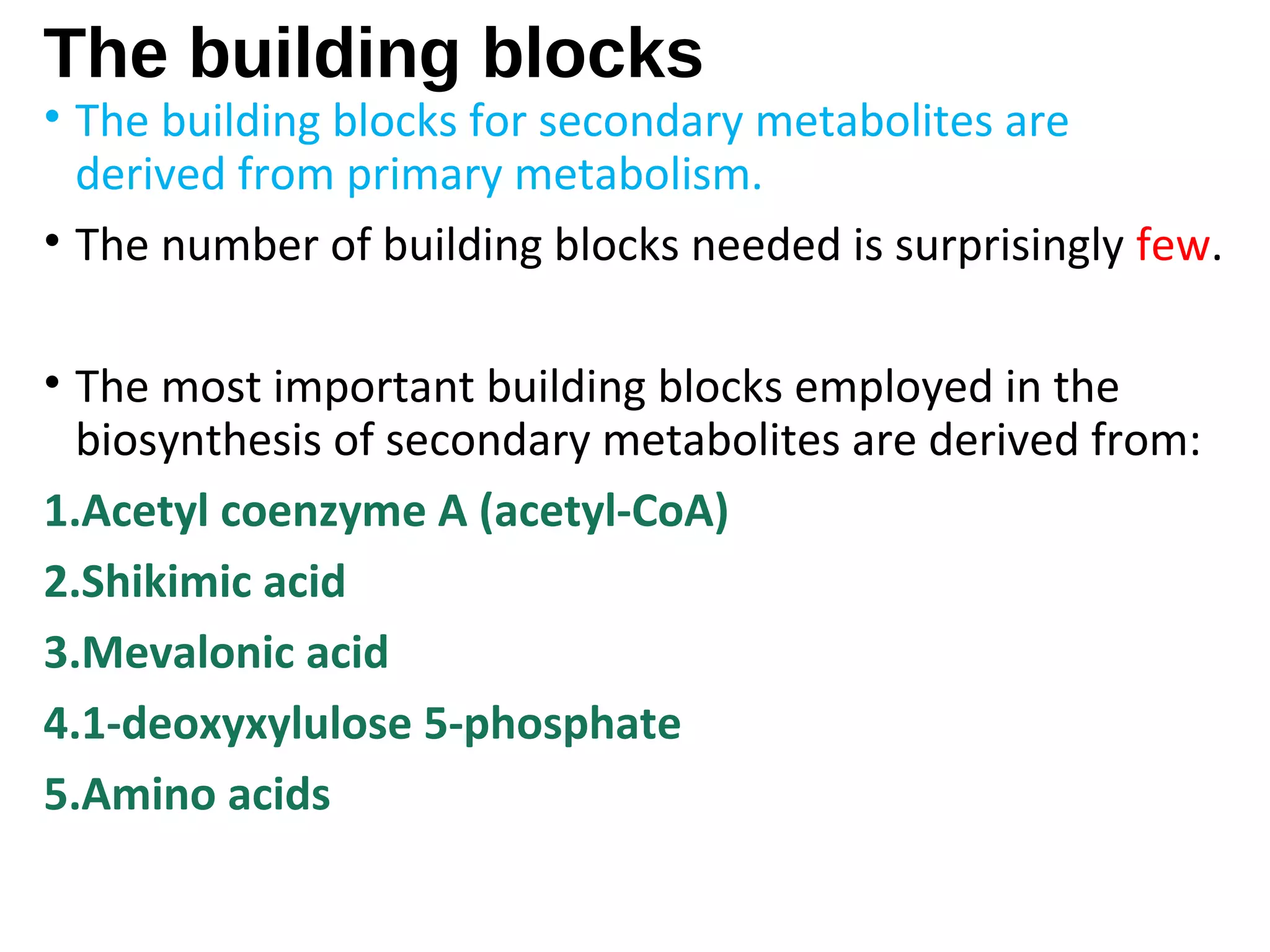
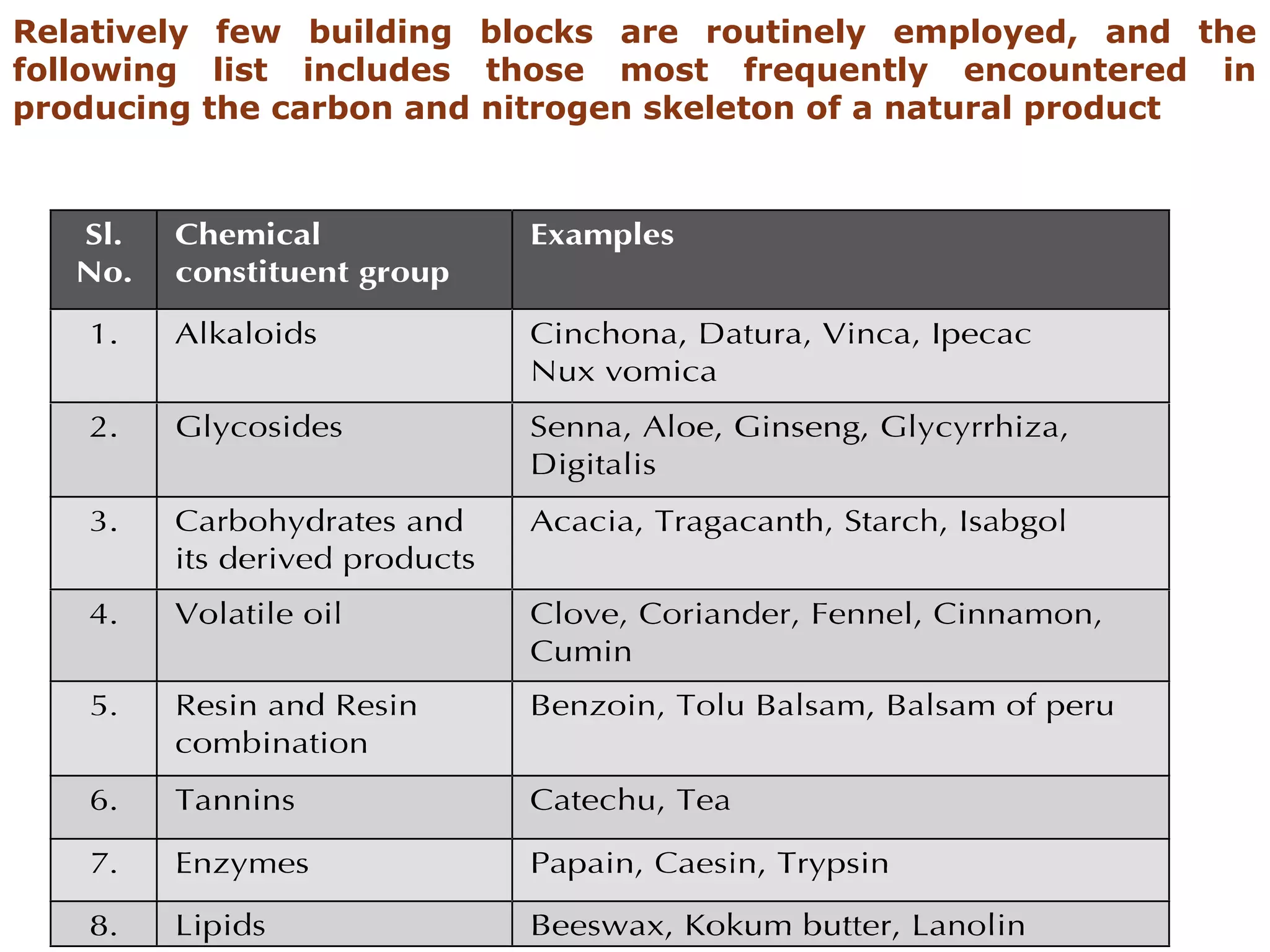
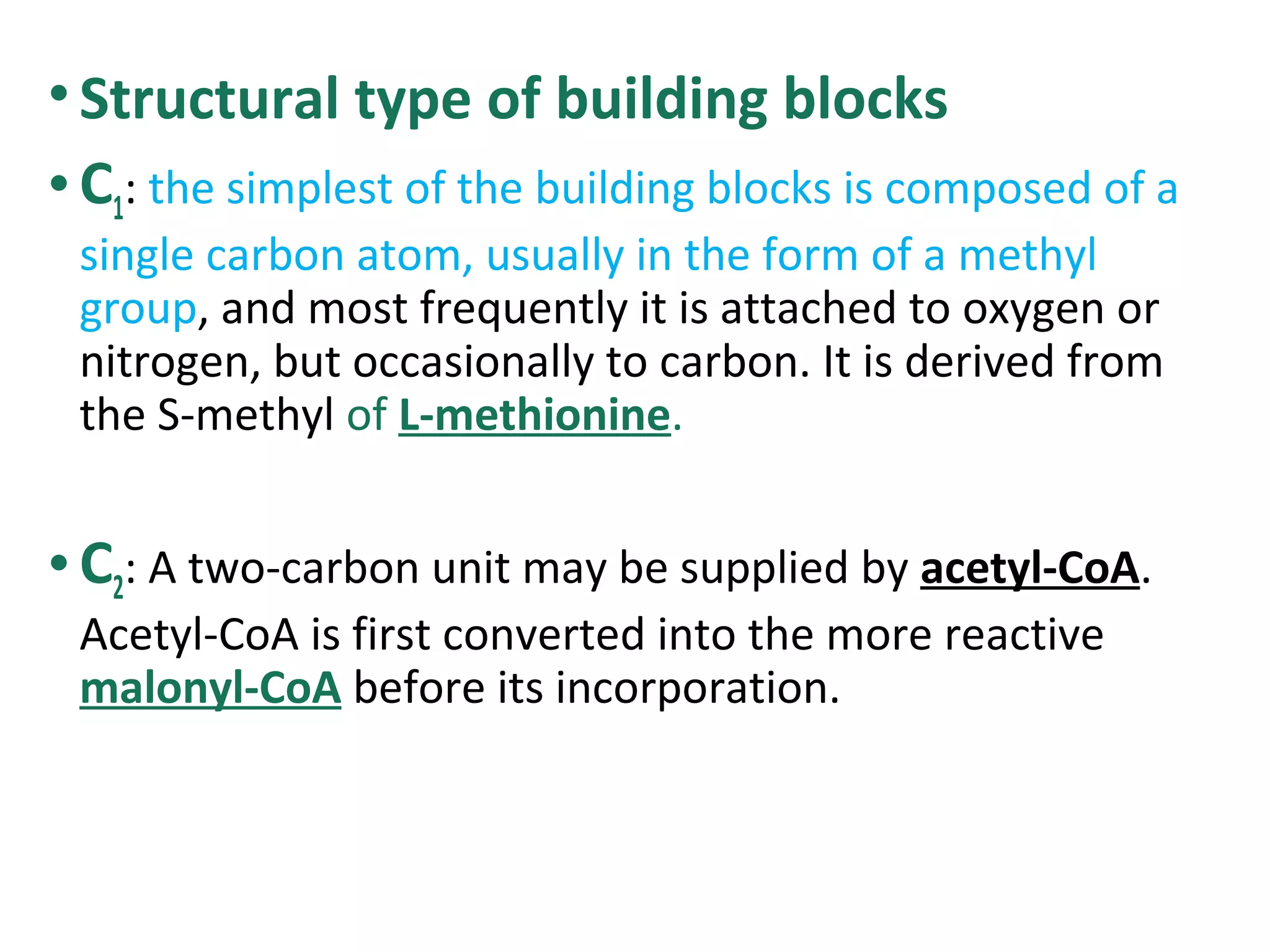
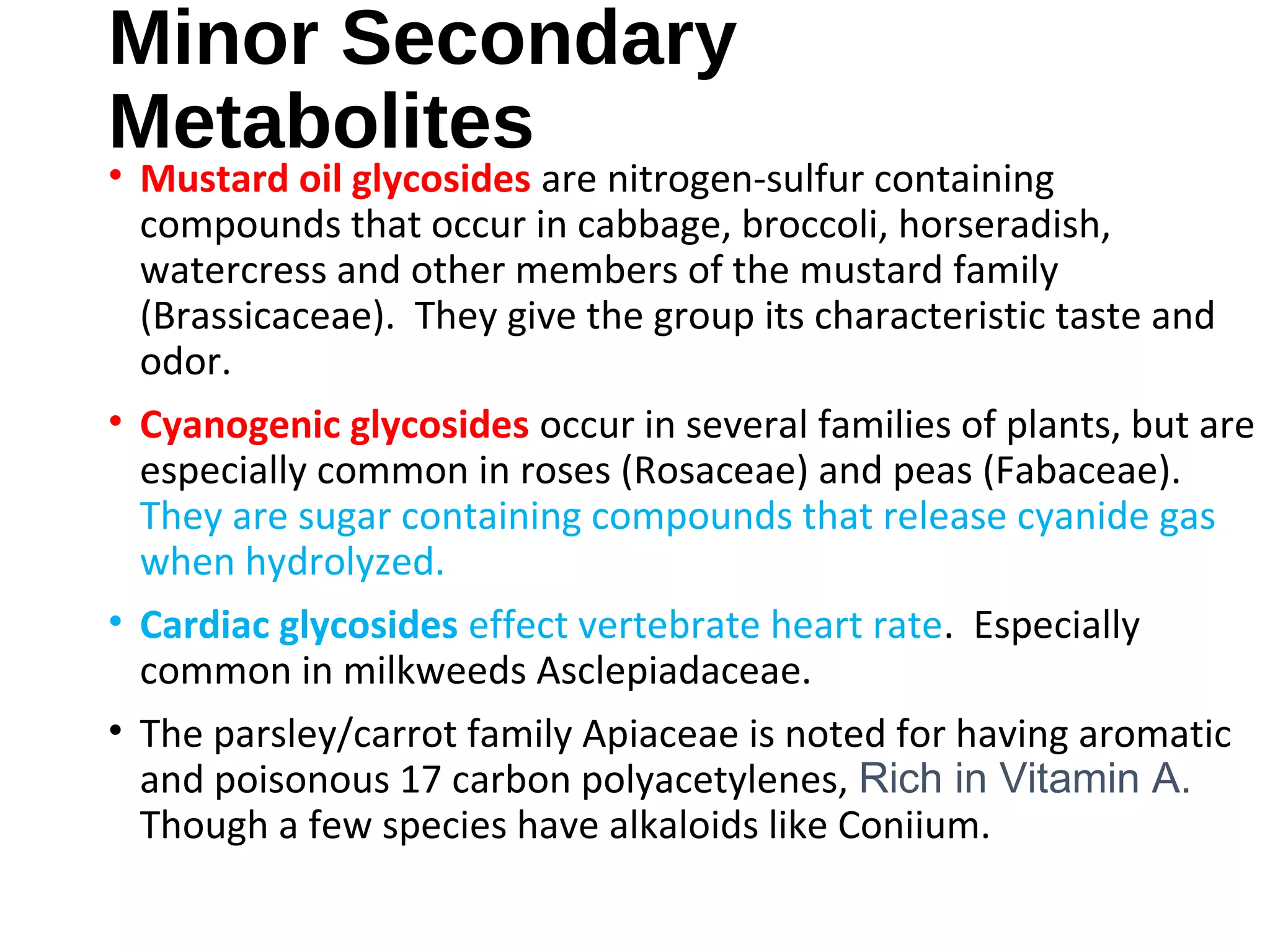
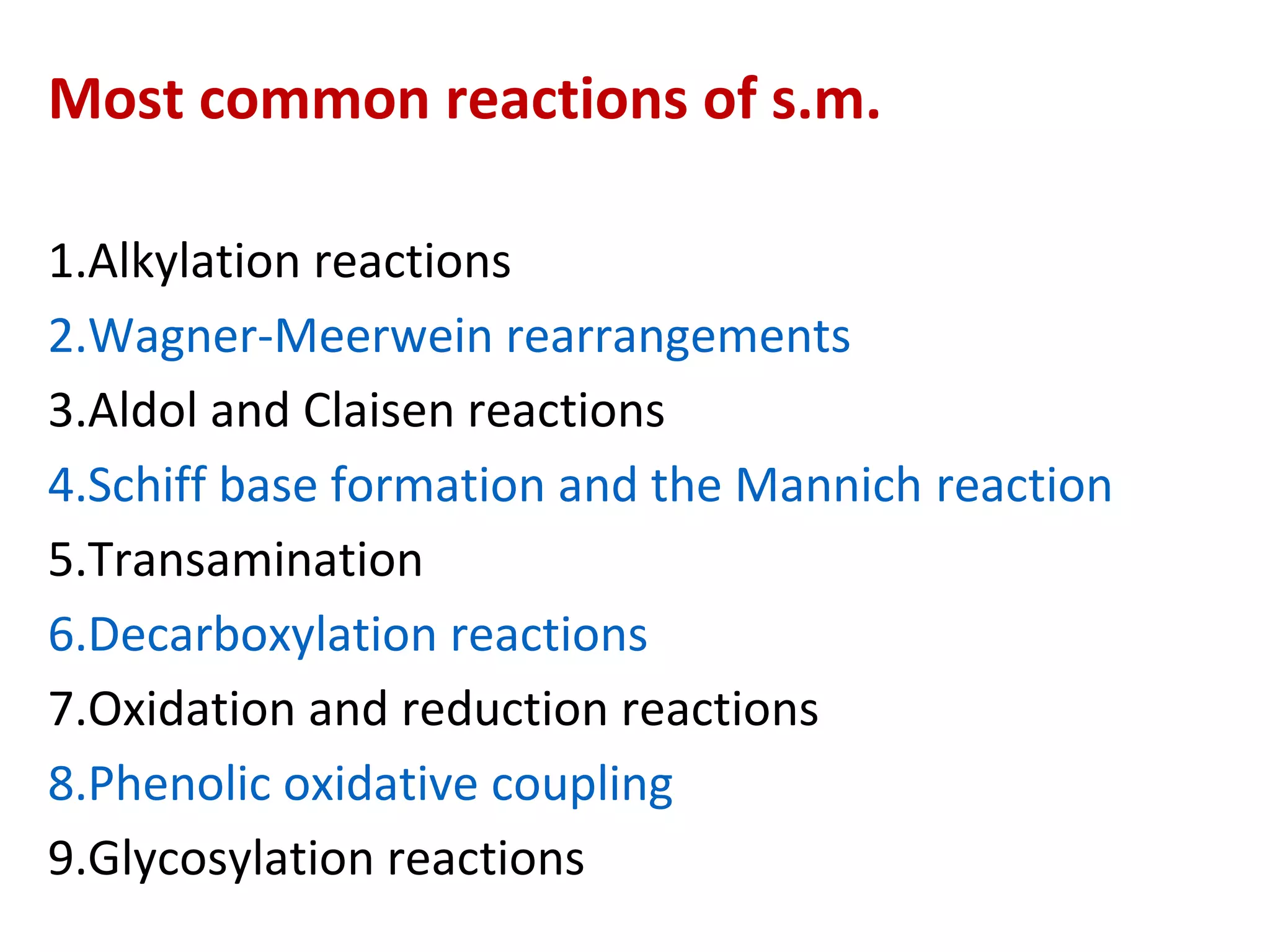
Secondary metabolism refers to metabolic pathways that are not essential for growth, development or reproduction in organisms. Secondary metabolites are compounds produced by these pathways that usually have ecological functions. There are a few key building blocks that contribute to secondary metabolite production, including acetate, shikimate acid, mevalonate, and amino acids. Secondary metabolites play important roles in plant defense against predators and attraction of pollinators, and are classified based on their structures and biosynthetic pathways.