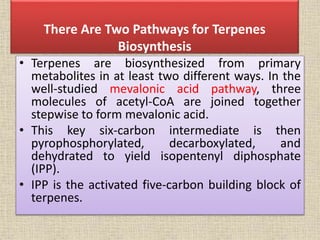
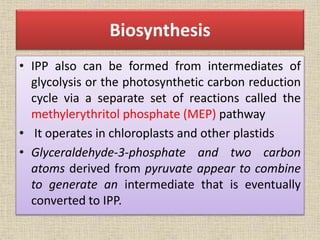
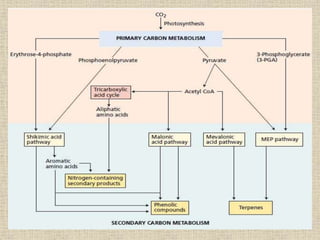
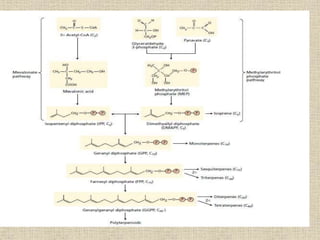
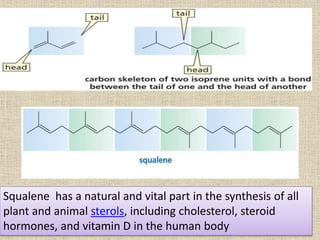
This document discusses terpenes, a class of plant secondary metabolites. It begins by defining secondary metabolites and dividing them into three major groups, with terpenes being the largest group. Terpenes are formed from fused five-carbon isoprene units and are classified based on this number, with monoterpenes having two units. There are two pathways for terpene biosynthesis, one involving mevalonic acid and the other methylerythritol phosphate. Important terpenes include lycopene, beta-carotene, and squalene. Terpenes have various biological effects and historical economic uses, such as menthol in medicines and fragrances.


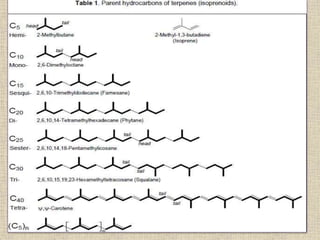
![CLASSIFICATION OF TERPENES
• Terpenes are classified by the number of five-
carbon units they contain.
• Ten-carbon terpenes, which contain two C5 units,
are called monoterpenes
• 15-carbon terpenes (three C5 units) are
sesquiterpenes
• 20-carbon terpenes (four C5 units) are
diterpenes.
• Larger terpenes include triterpenes (30 carbons),
tetraterpenes (40 carbons),and poly terpenoids
([C5]n carbons, where n > 8).](https://image.slidesharecdn.com/terpenes2-170209133340/85/Terpenes-7-320.jpg)