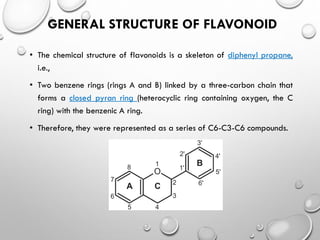
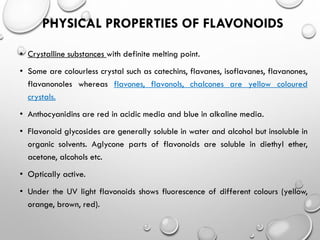
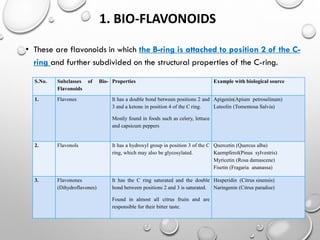
Flavonoids are phytochemical compounds found in various plants, recognized for their distinct structures based on a diphenyl propane skeleton. They are classified into bio-flavonoids, iso-flavonoids, and neo-flavonoids, each having unique properties and biological sources. Flavonoids serve essential roles in plants, including attracting pollinators, providing color and aroma, and exhibiting antioxidant and protective functions.