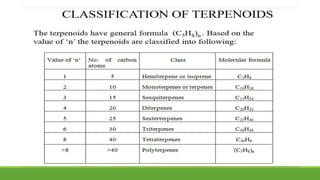
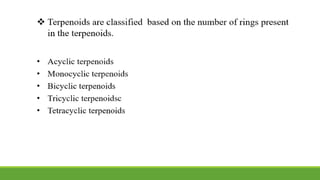
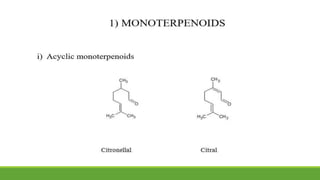
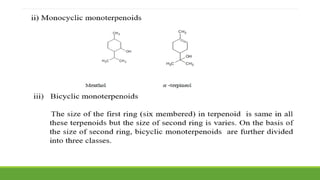
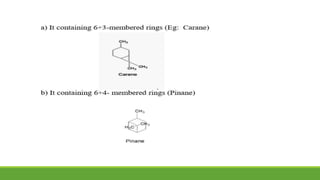
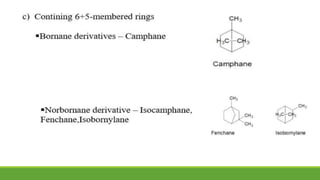
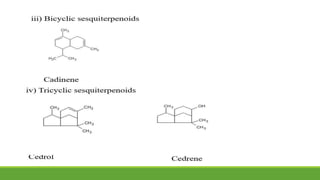
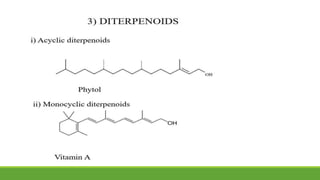
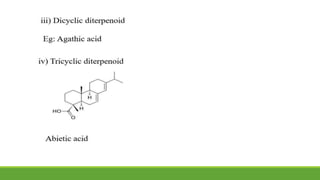
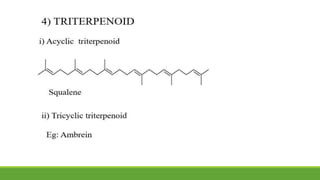
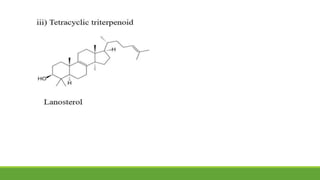
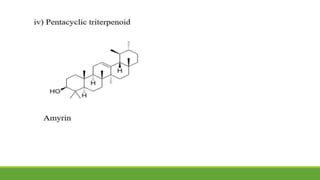
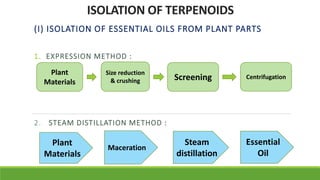
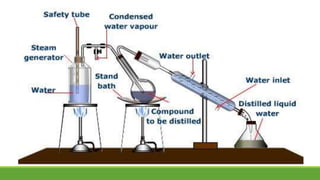
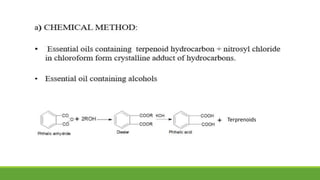
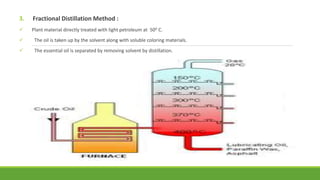
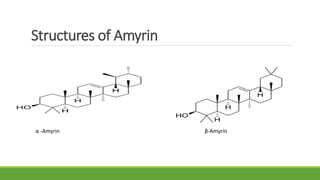
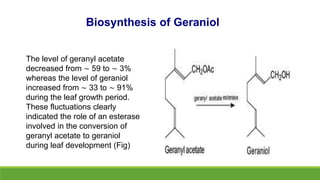
Group A presented on terpenoids, a large class of organic compounds found in plants composed of linked isoprene units. Terpenoids have unsaturated and volatile properties and represent the essential oils and active constituents of plants. They undergo various chemical reactions and can be isolated from plants through steam distillation or expression. Specific terpenoids discussed include amyrin, geraniol, and their importance. Terpenoids have many applications in perfumes, cosmetics, foods, and pharmaceuticals due to their therapeutic properties such as being antiseptic, analgesic, and insect repellents.