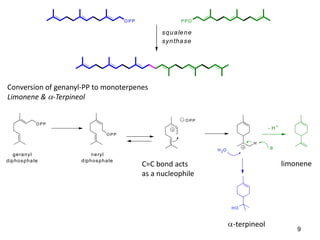
1) Terpenes are derived from head-to-tail polymerization of isoprene units (C5). Monoterpenes contain 2 units, sesquiterpenes contain 3 units, and so on.
2) Mevalonic acid is converted to isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), the basic C5 units. Repeated condensation of these units leads to prenyl diphosphates of different sizes.
3) These prenyl diphosphates are converted by terpene synthases into the skeletons of various terpenes, which may then undergo further modifications. This