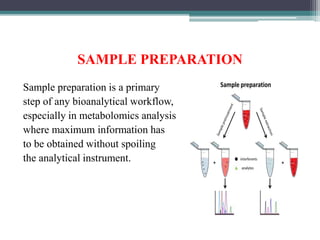
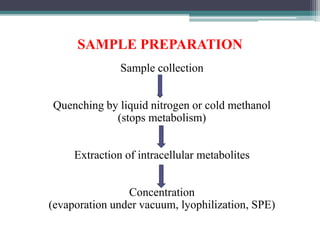
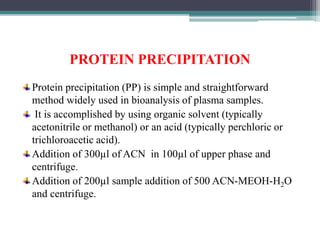
This document outlines the critical role of sample preparation in metabolite identification for bioanalytical workflows, emphasizing techniques like liquid-liquid extraction, protein precipitation, and solid phase extraction. It details various methods and protocols for sample collection and preparation aimed at ensuring accurate metabolomics analysis, including in vivo sampling techniques and modern extraction approaches. Additionally, it highlights the importance of choosing appropriate sample preparation methods to enhance metabolite coverage and maintain biological relevance in the data obtained.