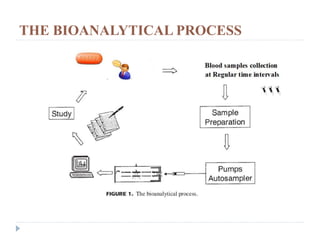
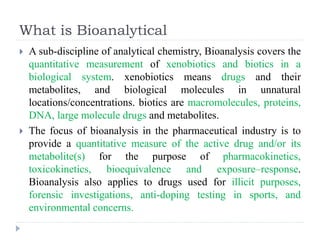
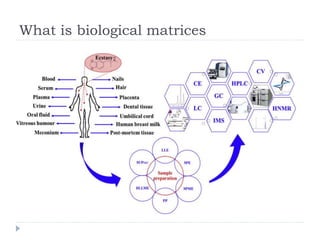
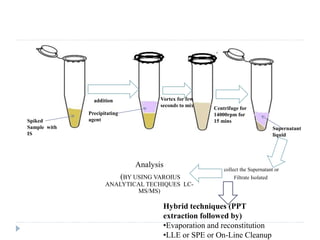
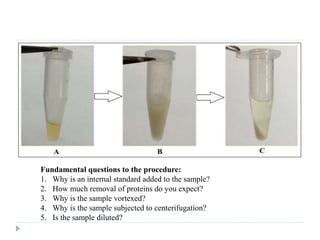
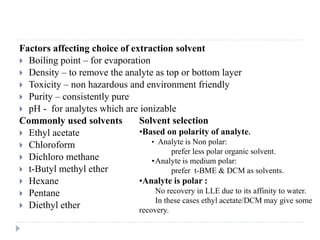
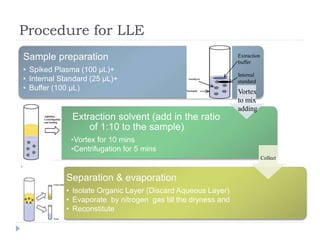
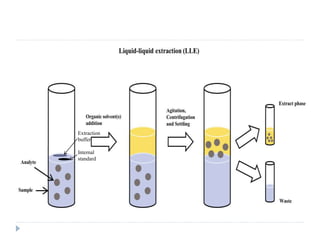
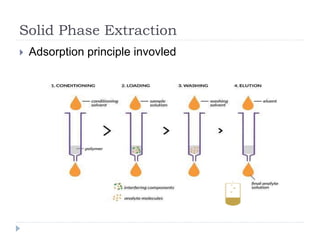
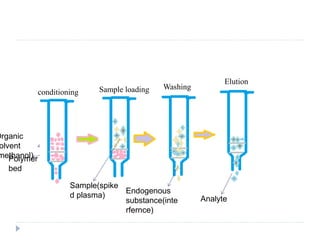
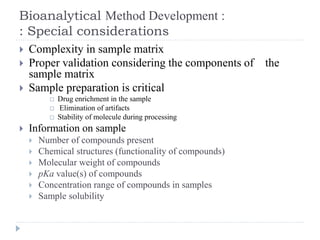
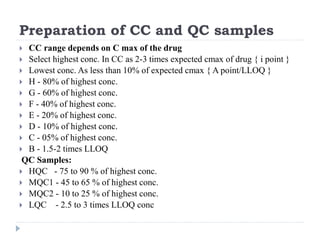
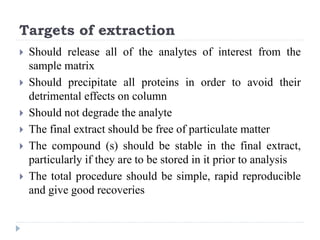
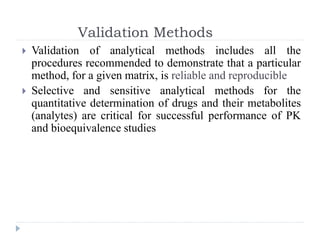
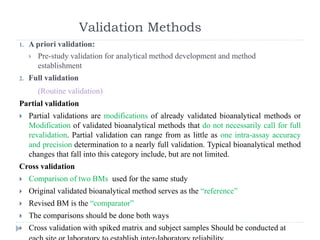
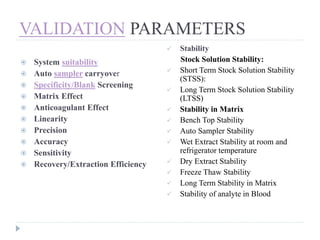
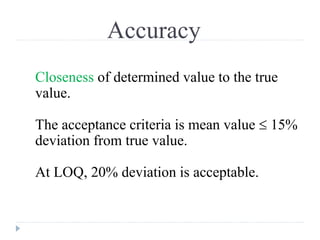
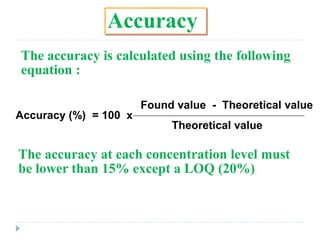
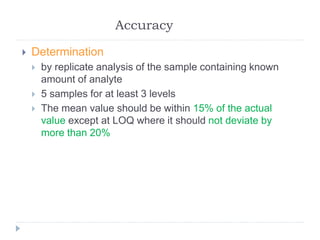
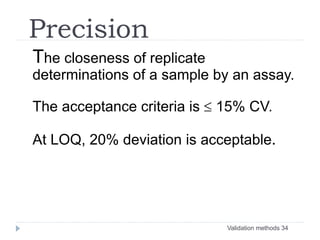
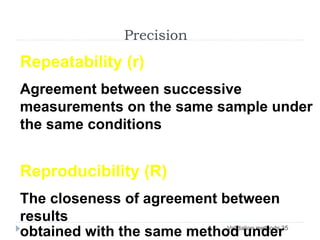
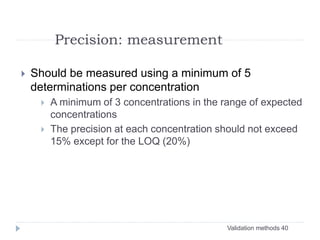
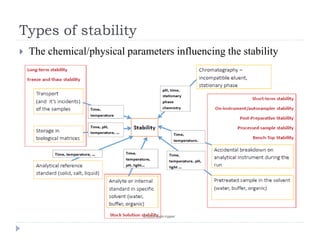
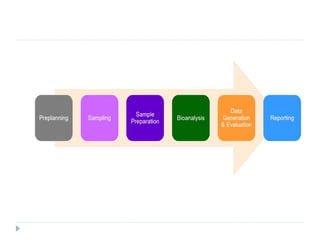
The document discusses bioanalytical methods for quantifying drugs and metabolites in biological samples. It covers various sample preparation techniques including protein precipitation, liquid-liquid extraction, and solid phase extraction. Protein precipitation is commonly used to remove proteins from samples by adding precipitating agents like acids or organic solvents. Liquid-liquid extraction partitions analytes between two immiscible solvents, while solid phase extraction uses an adsorbent material to separate analytes from the sample matrix. Validation of bioanalytical methods assesses parameters like accuracy, precision, selectivity, sensitivity and stability.