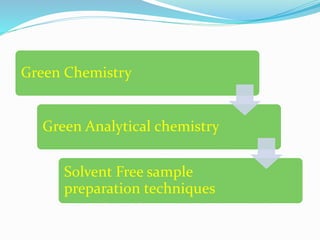
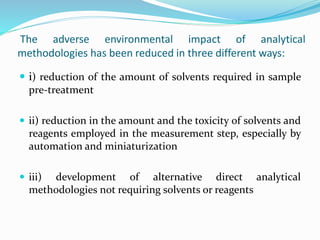
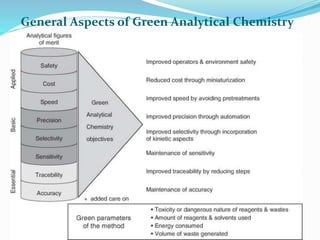
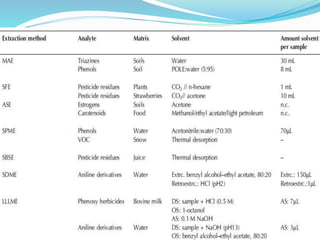
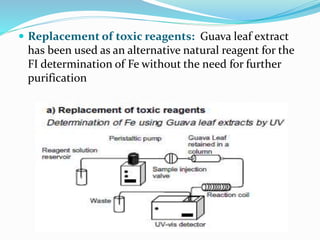
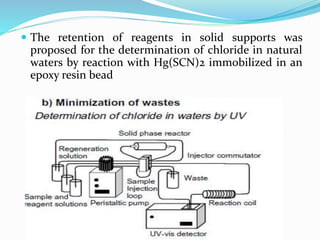
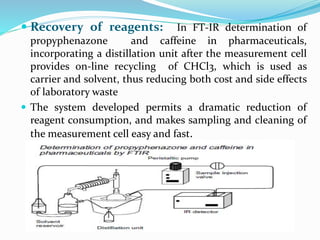
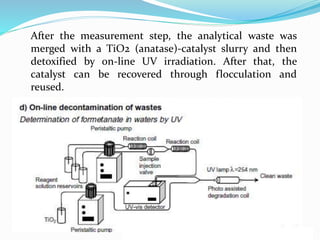
This document discusses green analytical chemistry and ways to make analytical methods more environmentally friendly. It describes principles of green chemistry and how they can be applied to analytical techniques. Some ways to make methods greener include reducing solvent and reagent use through miniaturization, automation, direct analysis of samples, and online recycling or decontamination of waste. Specific greener sample pretreatment and analytical techniques are also outlined, such as microwave-assisted extraction, solid-phase microextraction, and Fourier transform infrared spectroscopy for solvent-free determinations. The overall goal is to minimize environmental impacts and hazards of chemical analysis.