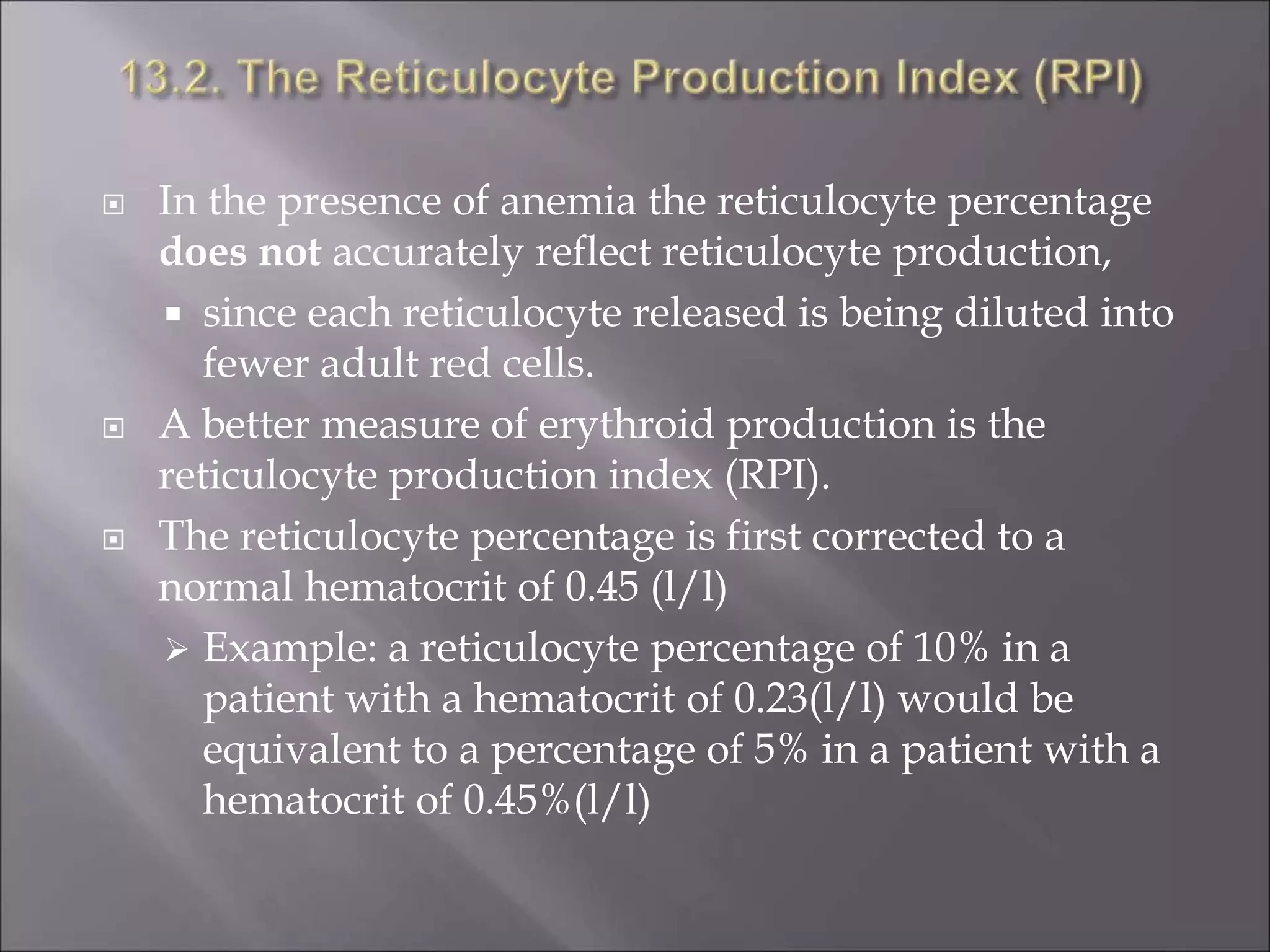
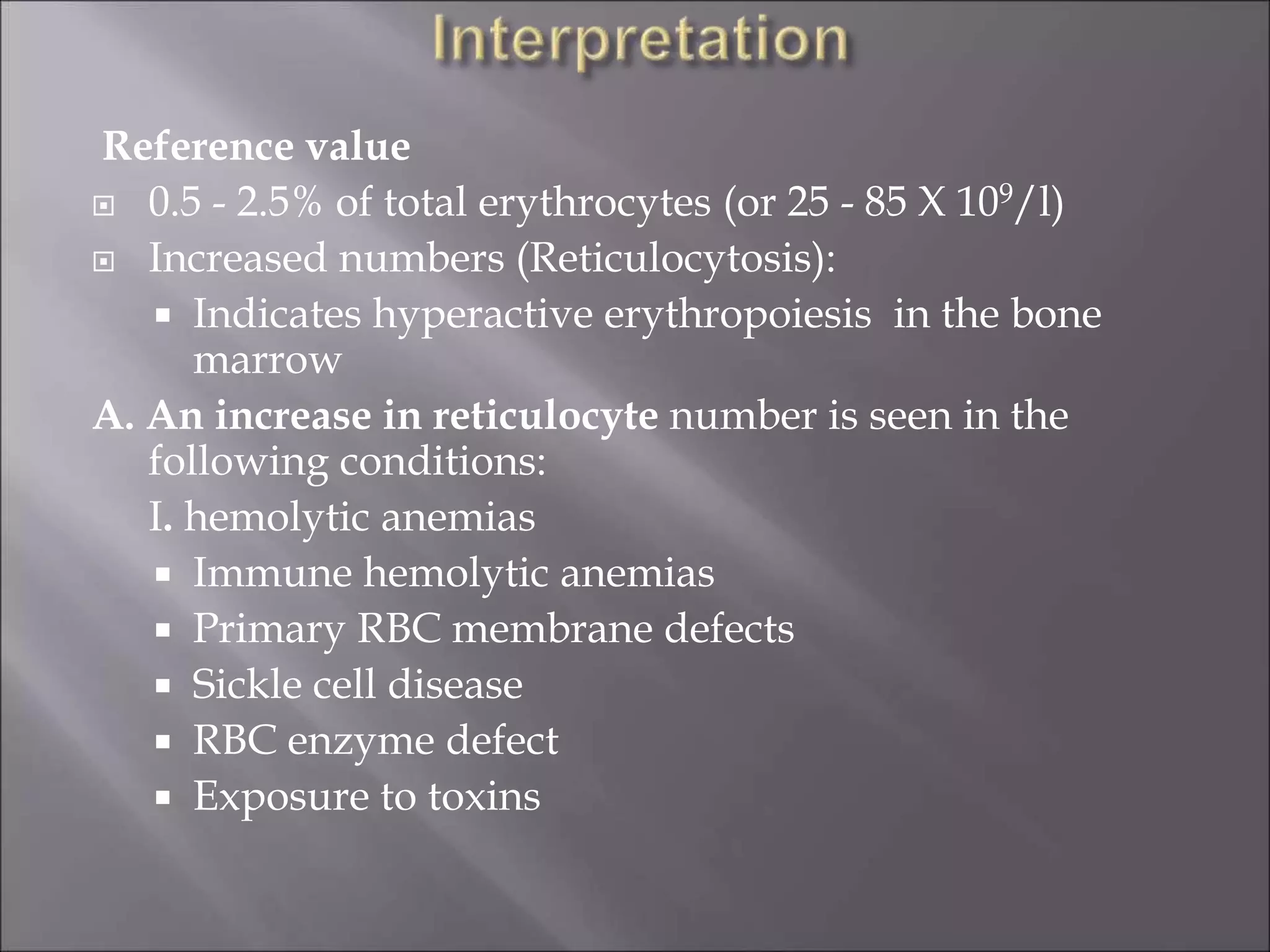
Reticulocytes are immature red blood cells that contain remnants of ribosomal RNA. Their number in peripheral blood reflects bone marrow erythropoietic activity. Supravital staining allows viable reticulocyte counting by staining their RNA blue. Reticulocyte production index (RPI) corrects the count for anemia severity and indicates marrow function. Increased reticulocytes suggest effective marrow response to anemia or blood loss, while low levels may mean marrow damage or ineffective erythropoiesis. Proper technique and quality controls are needed to obtain accurate counts.