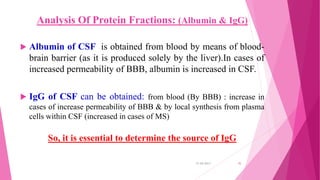
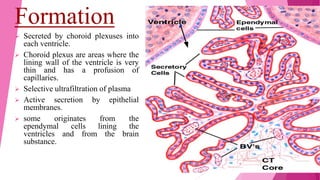
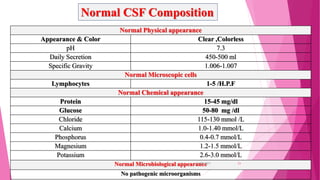
Cerebrospinal fluid (CSF) is a clear, colorless fluid produced primarily by the choroid plexus in the brain's ventricles, serving critical functions like cushioning the brain, nutrient transport, and waste removal. It circulates through the brain and spinal cord, with key clinical applications in diagnosing conditions such as meningitis, encephalitis, and cancers. Routine analysis of CSF involves collection via lumbar puncture and assessment of its physical, chemical, and cellular compositions to detect abnormalities.





![CIRCULATION OF CSF
Lateral ventricle
Foramen of Monro [Interventricular foramen]
Third ventricle:
Subarachnoid space of Brain and Spinal cord
Fourth ventricle:
Cerebral aqueduct
Foramen of megendie and formen of luschka
31-05-2017 10](https://image.slidesharecdn.com/cerebrospinalfluid-170610160703/85/Cerebrospinal-fluid-7-320.jpg)












![MULTIPLE SCLEROSIS
CSF protein is normal or mildly increased.
Increased IgG in CSF, but not in serum [IgG/albumin index normally
10:1].
90% of MS patients have oligoclonal IgG bands in the CSF.
Oligoclonal bands occur in the CSF only not in the serum.
The CSF in MS often contains myelin fragments and myelin basic
protein (MBP).
MBP can be detected by radioimmunoassay. MBP is not specific for
MS. It can appear in any condition causing brain necrosis, including
infarcts.
31-05-2017 32](https://image.slidesharecdn.com/cerebrospinalfluid-170610160703/85/Cerebrospinal-fluid-23-320.jpg)