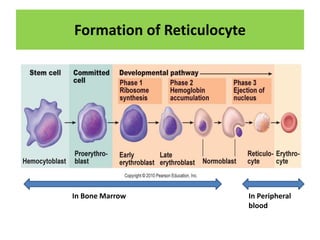
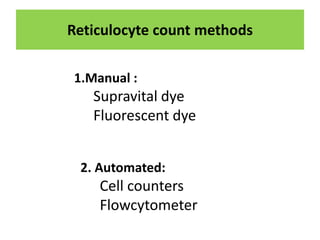
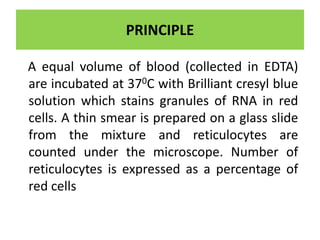
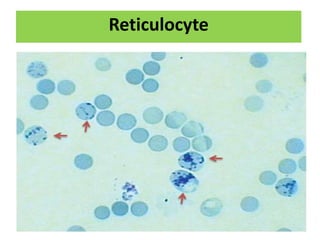
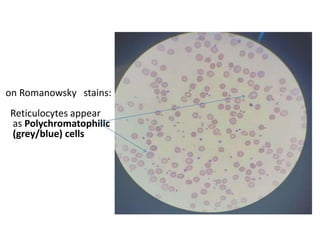
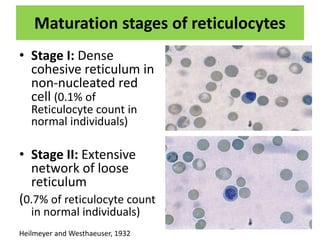
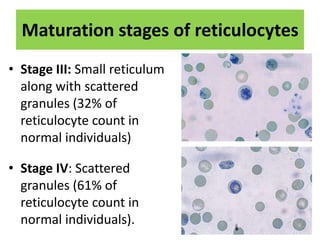
Reticulocytes are immature red blood cells that are released from the bone marrow into circulation. They contain remnants of RNA and ribosomes. Reticulocyte counts are used to assess bone marrow response to anemia and erythropoietin therapy. Reticulocytes can be manually counted using supravital dyes like new methylene blue that stain the RNA, or automatically using cell counters that detect nucleic acid content. Normal ranges are 0.5-2.5% and increased counts indicate bone marrow response to anemia or therapy, while decreased counts suggest bone marrow suppression.