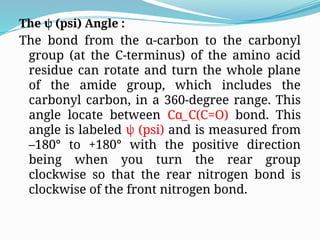
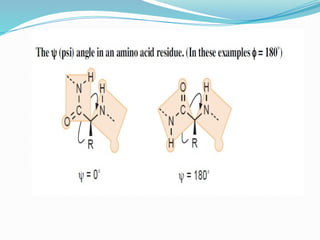
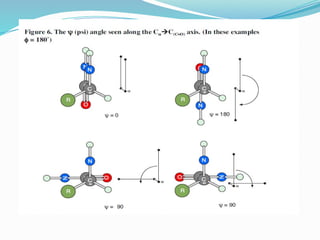
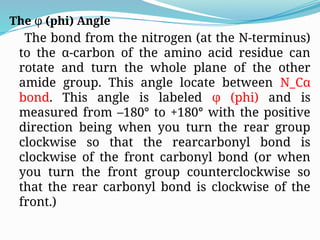
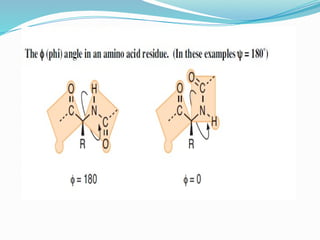
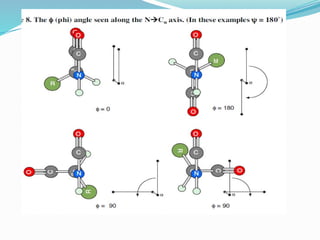
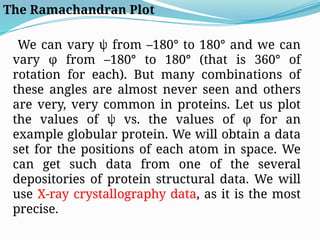
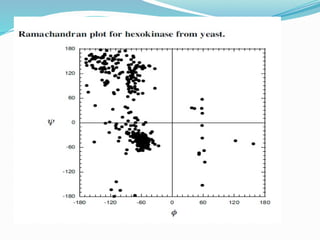
Could you clarify what you mean by "Ramchandra plot"? Are you referring to:
1. A plot (graph/diagram) related to a scientific concept or dataset (perhaps in physics or material science)?
2. A story or storyline involving Lord Ramchandra from Hindu mythology?
3. A land or property plot owned or related to someone named Ramchandra?
Let me know so I can help accordingly!
Thanks! Since "Ramchandra plot" could mean different things depending on context, here are some possible interpretations with a bit more detail:
1. Scientific or Mathematical Plot
There’s no widely recognized scientific graph or chart specifically called a "Ramchandra plot" in mainstream physics, chemistry, or material science.
It's possible you're referring to a plot or data visualization used or proposed by a researcher named Ramchandra. If you have a paper or topic in mind (e.g., neutron diffraction, magnetism, etc.), I could dig deeper.
2. Mythological Storyline (Ramayana)
If you’re referring to Ramchandra (another name for Lord Rama), then a Ramchandra plot could mean:
The plot/storyline of the Ramayana, the epic tale of Rama's journey, exile, war with Ravana, etc.
A condensed version or retelling of the events focusing on Rama's perspective.
3. Real Estate / Land Plot
In everyday Indian English, a "Ramchandra plot" might refer to:
A piece of land (property/plot) owned by or named after someone called Ramchandra.
Possibly a location like Ramchandra Plot, Nagpur or other place names that include "Plot" as part of an address or region.
4. Something Else?
It might also be from a specific book, movie, local reference, or niche academic topic.
If you can tell me which direction you’re going—scientific, mythological, geographical, or something else—I can give a much more targeted explanation or even visuals.
Thanks! Since "Ramchandra plot" could mean different things depending on context, here are some possible interpretations with a bit more detail:
1. Scientific or Mathematical Plot
There’s no widely recognized scientific graph or chart specifically called a "Ramchandra plot" in mainstream physics, chemistry, or material science.
It's possible you're referring to a plot or data visualization used or proposed by a researcher named Ramchandra. If you have a paper or topic in mind (e.g., neutron diffraction, magnetism, etc.), I could dig deeper.
2. Mythological Storyline (Ramayana)
If you’re referring to Ramchandra (another name for Lord Rama), then a Ramchandra plot could mean:
The plot/storyline of the Ramayana, the epic tale of Rama's journey, exile, war with Ravana, etc.
A condensed version or retelling of the events focusing on Rama's perspective.
3. Real Estate / Land Plot
In everyday Indian English, a "Ramchandra plot" might refer to:
A piece of land (property/plot) owned by or named after someone called Ramchandra.
Possibly a location like Ramchandra Plot, Nagpur or other place names that include "Plot" as part of an address or regio