This document discusses several chemical reactions and concepts:
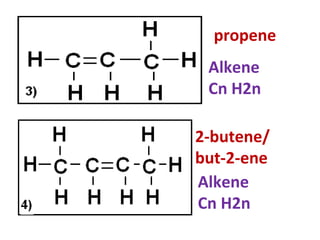
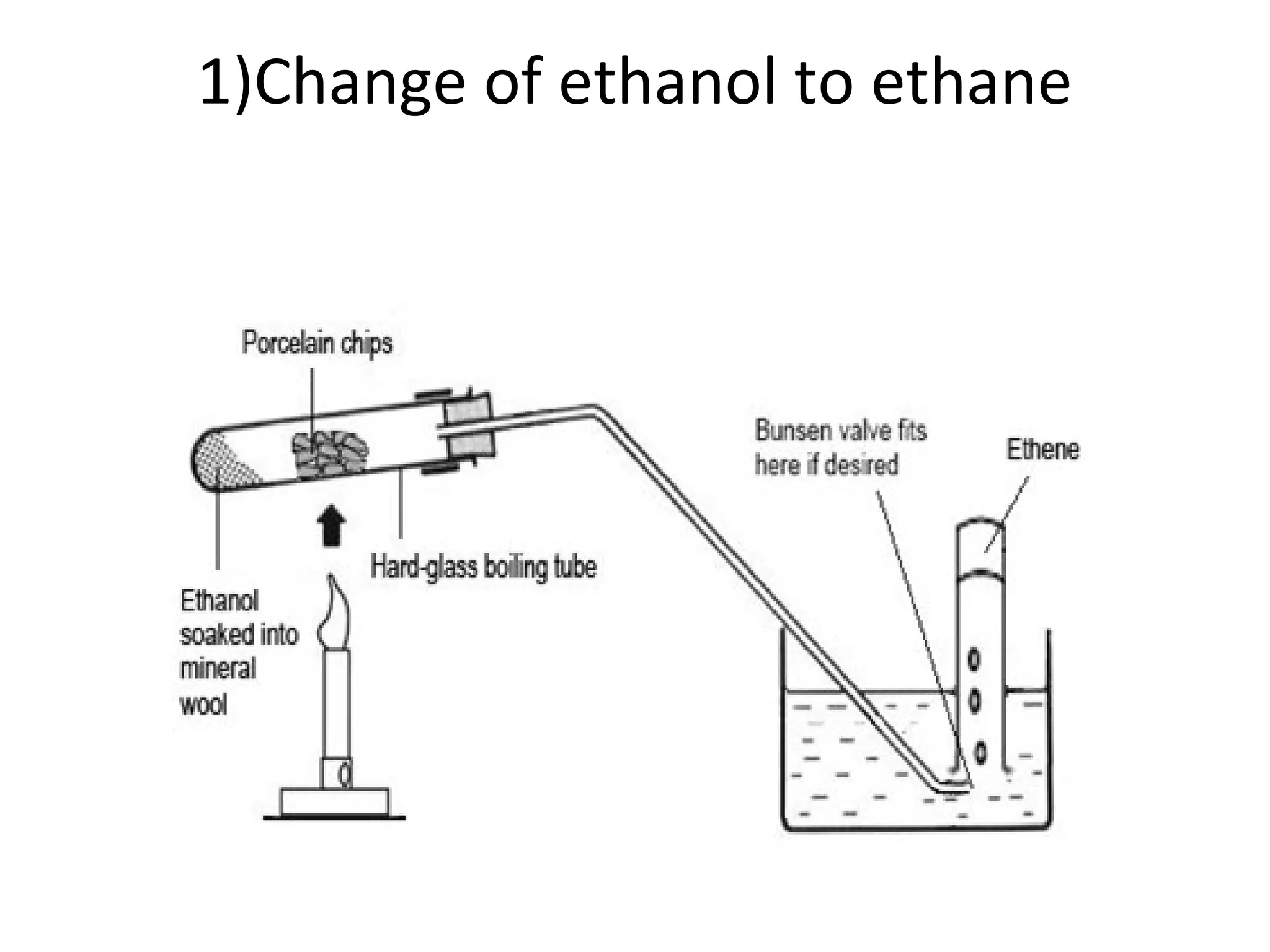
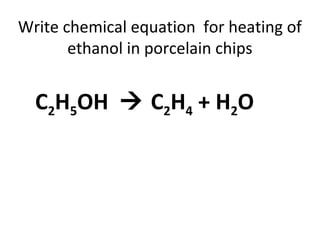
1) It describes the dehydration of ethanol to produce ethene, and the test to identify ethene using bromine.
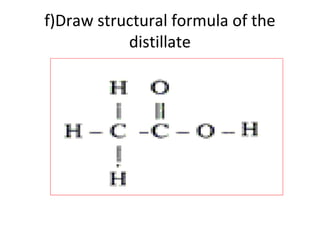
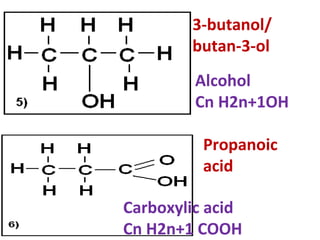
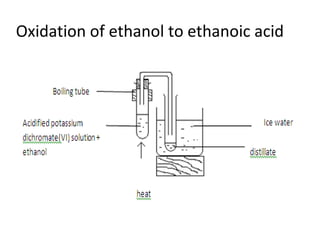
2) It discusses the oxidation of ethanol to produce ethanoic acid, including the chemical equation and properties of ethanoic acid.
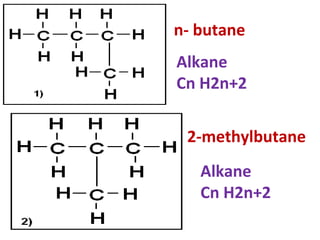
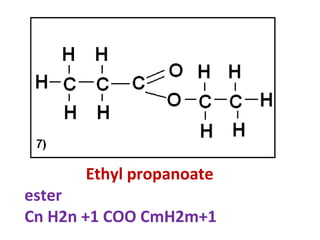
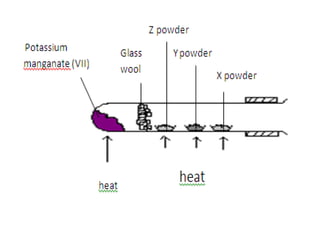
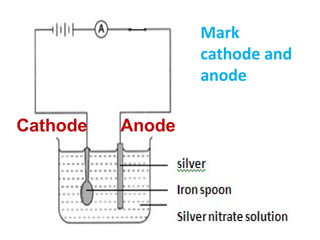
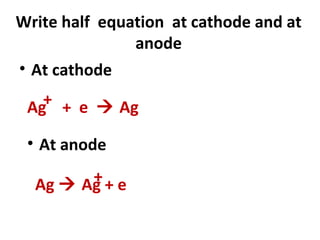
3) It covers several homologous series including alkanes, alkenes, alcohols, carboxylic acids, and esters.


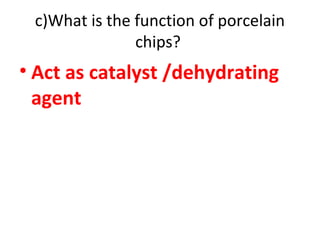





![d) Write chemical equation
C2H5OH + 2[O]
CH3COOH+ H2O](https://image.slidesharecdn.com/questionsdiagramset1terkini-1-101110213104-phpapp01/85/Questions-diagram-set-1-terkini-1-12-320.jpg)