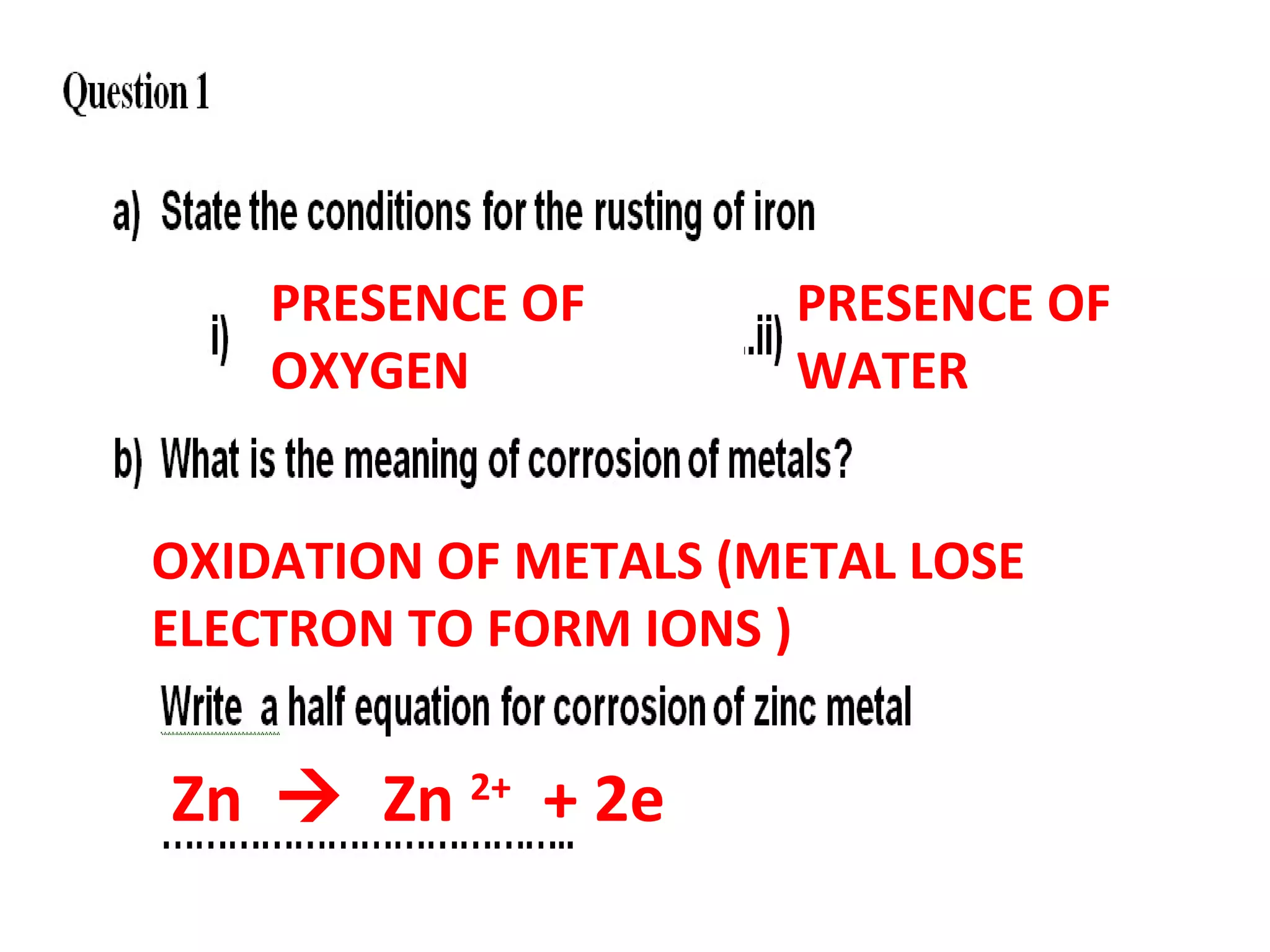
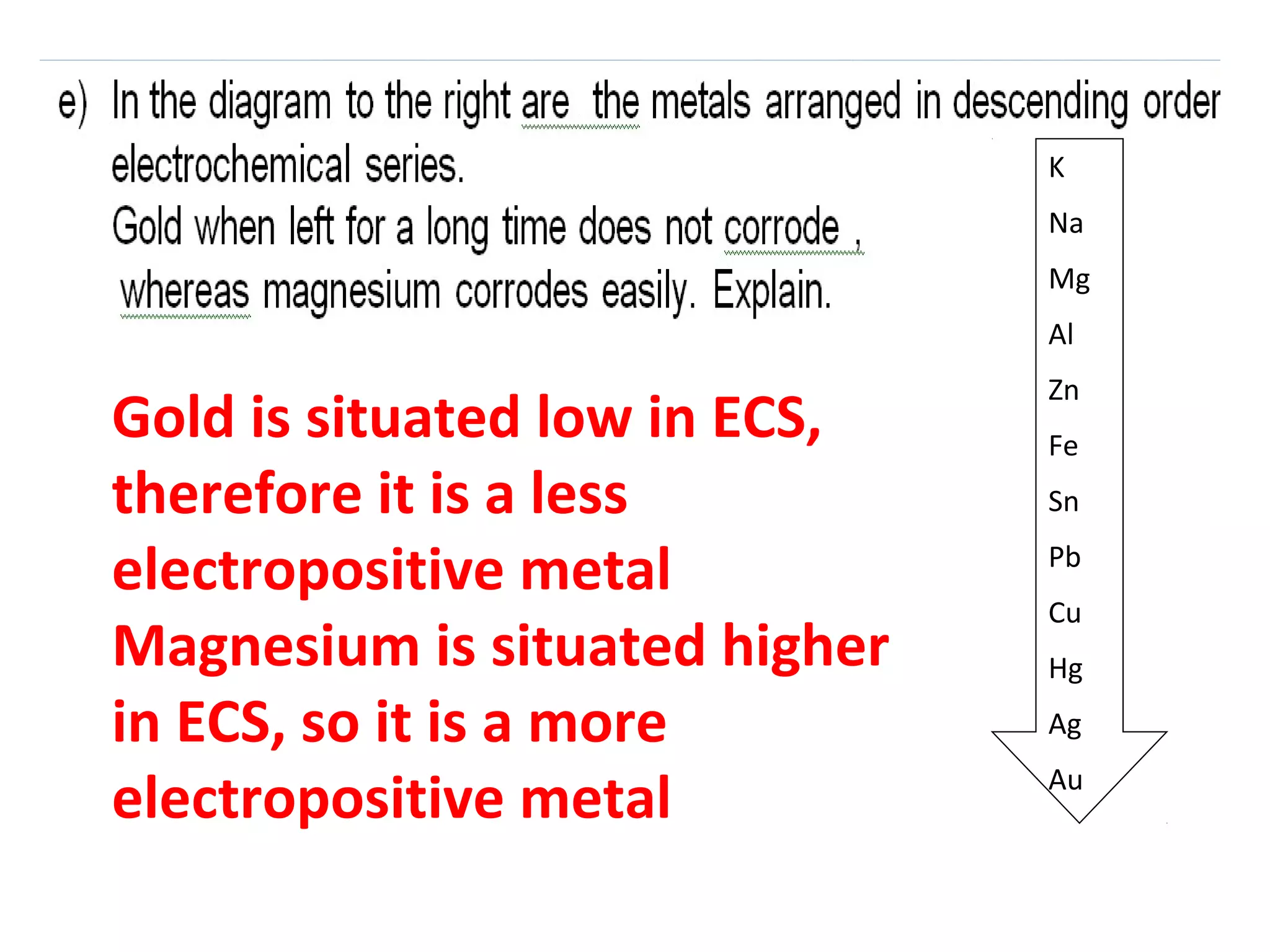
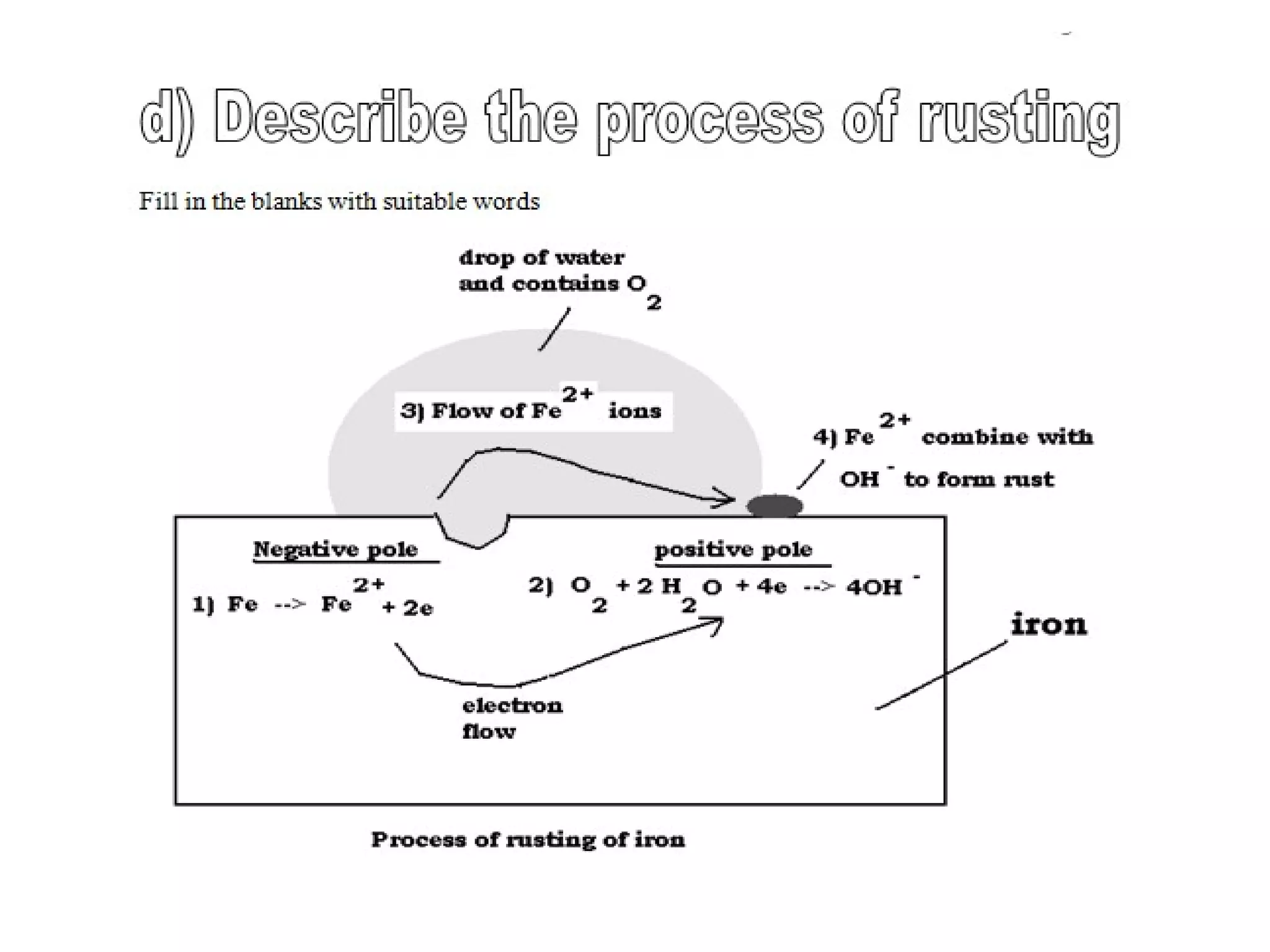
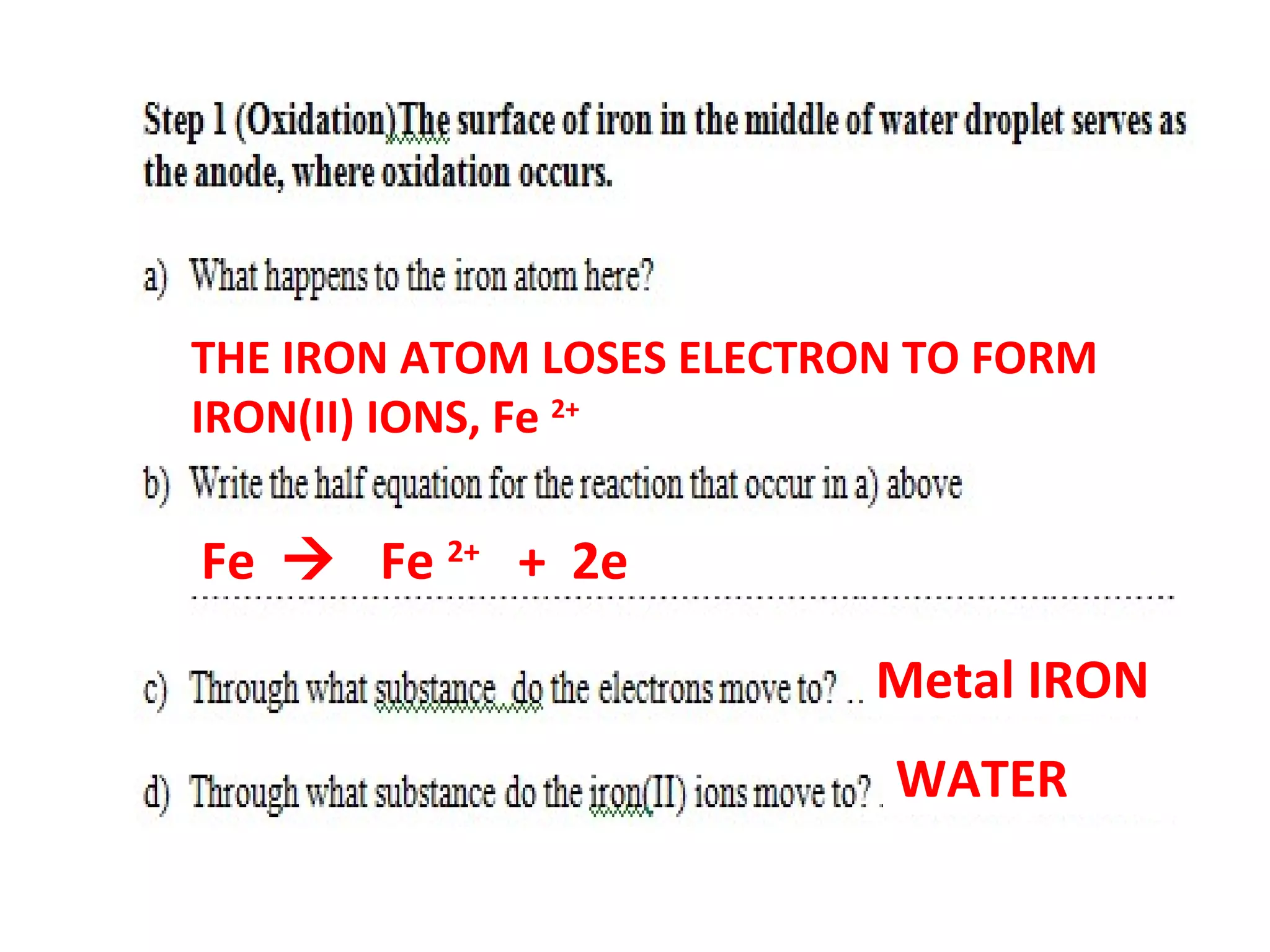
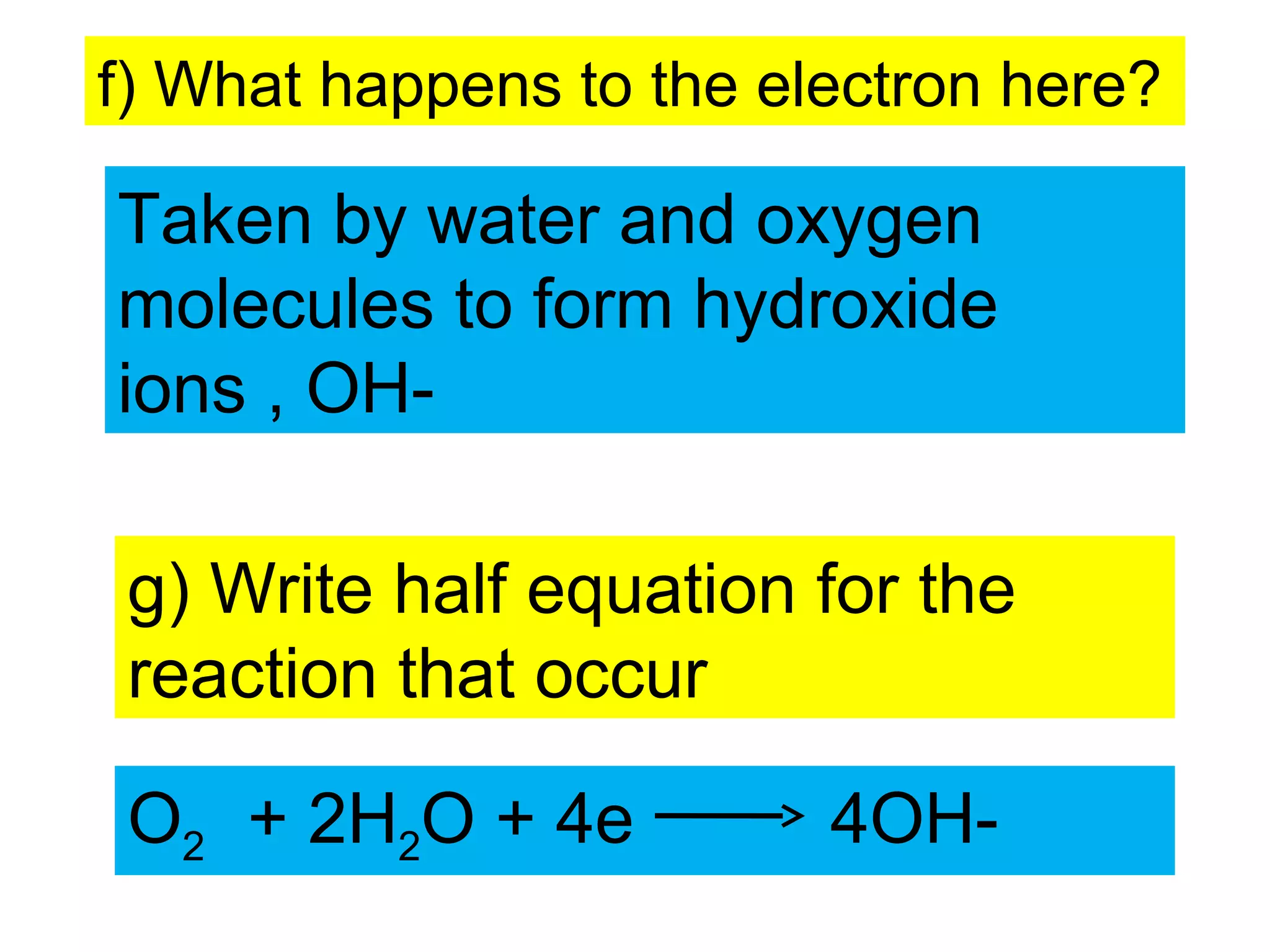
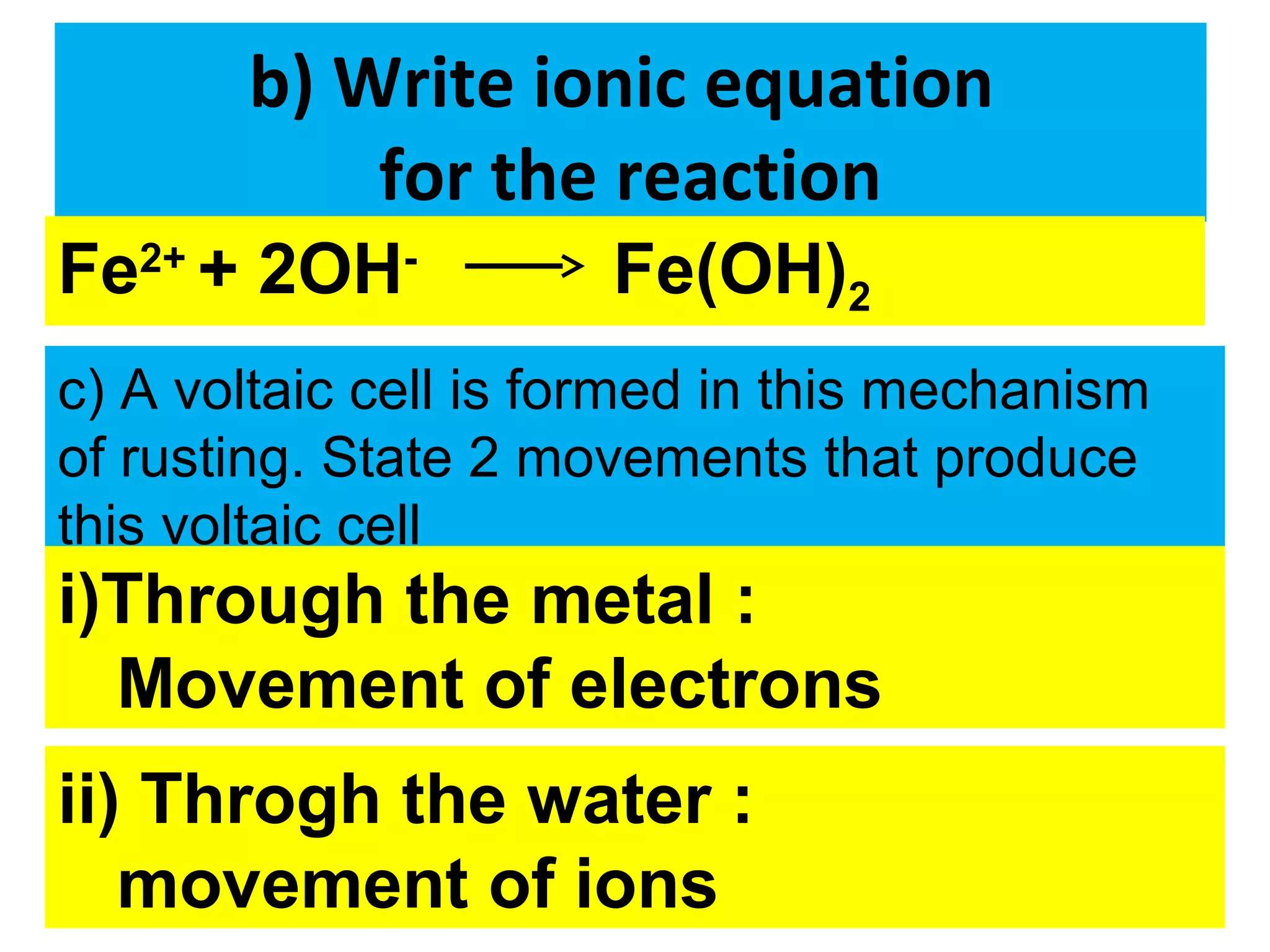
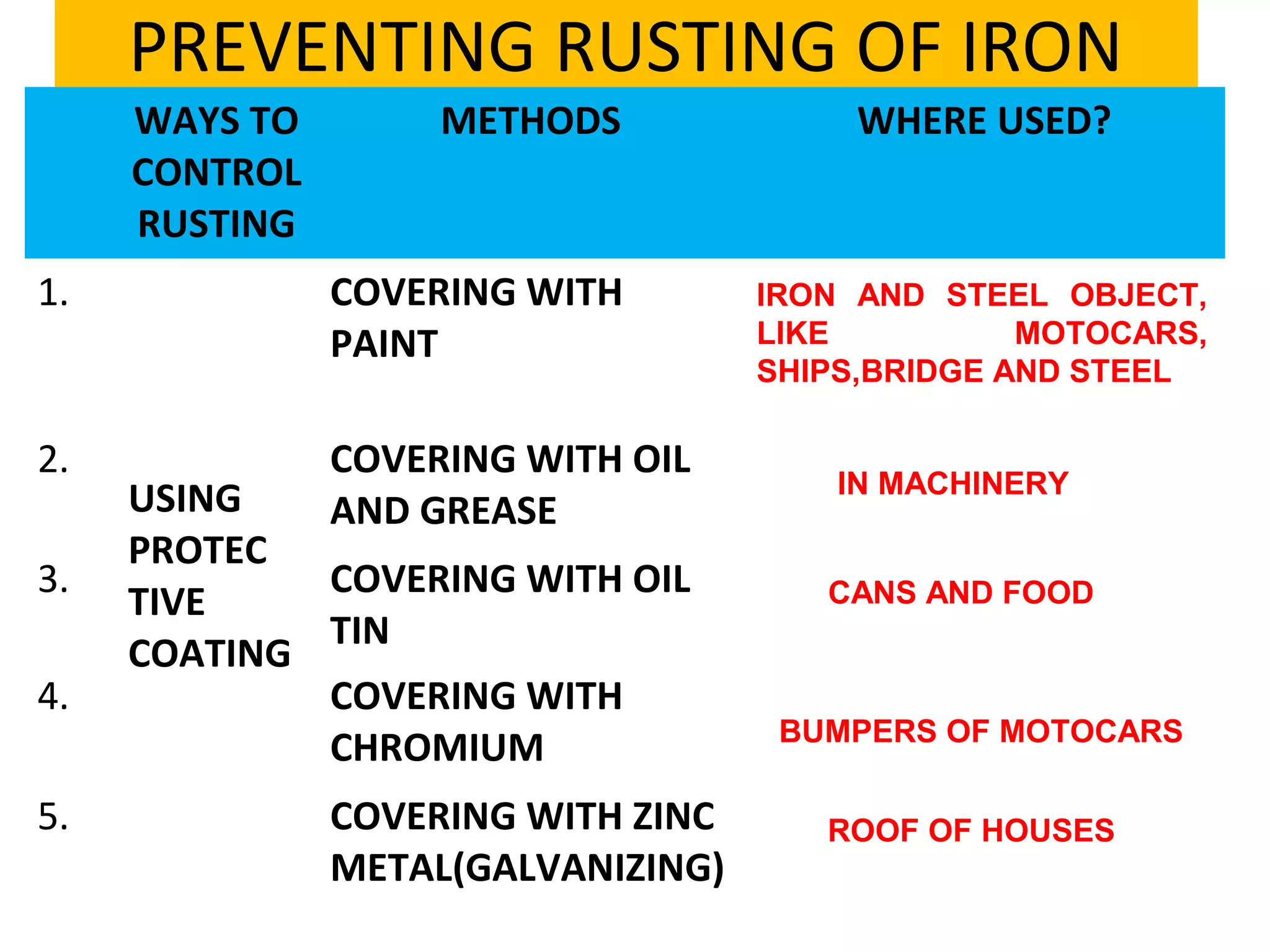
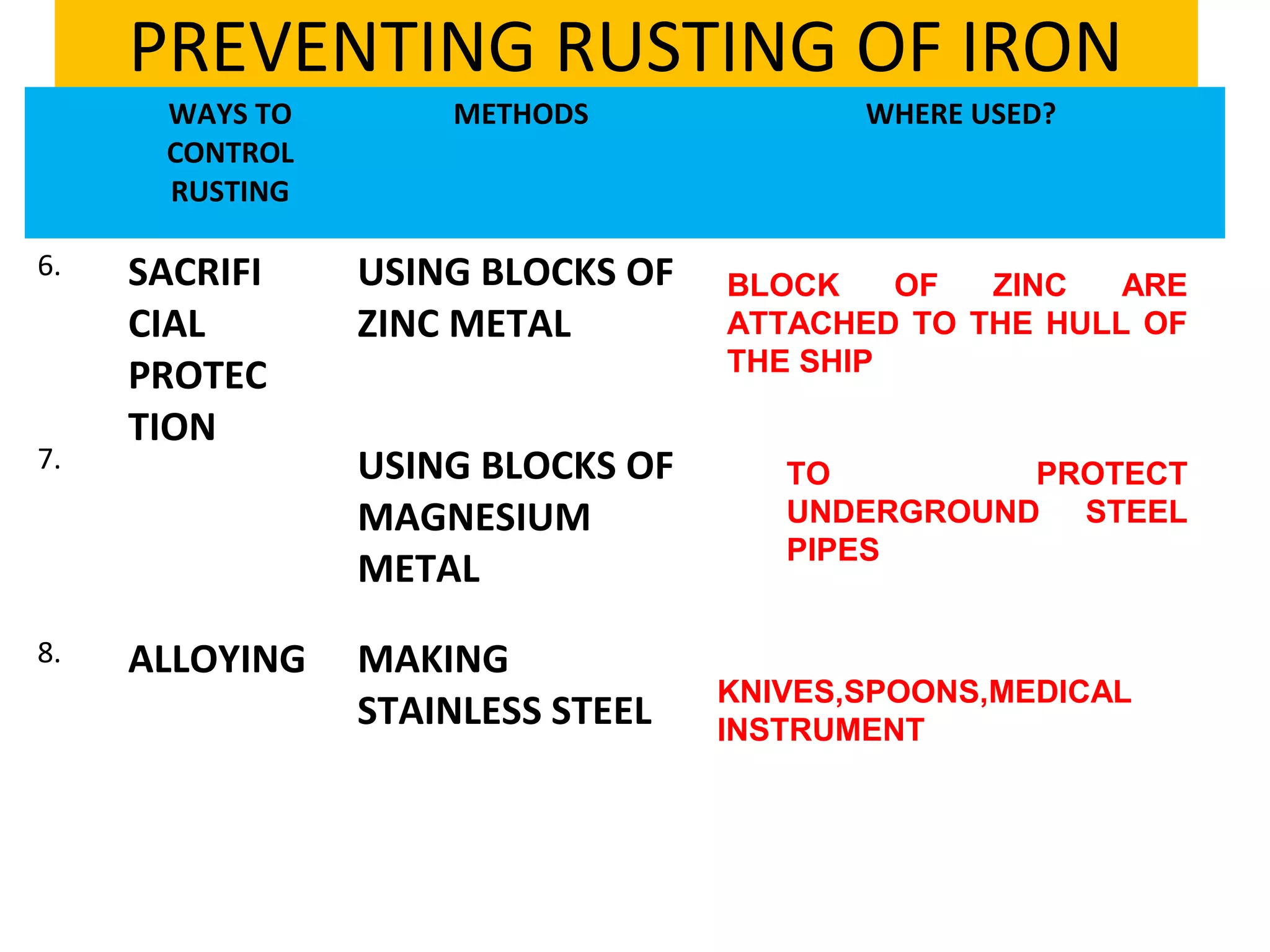
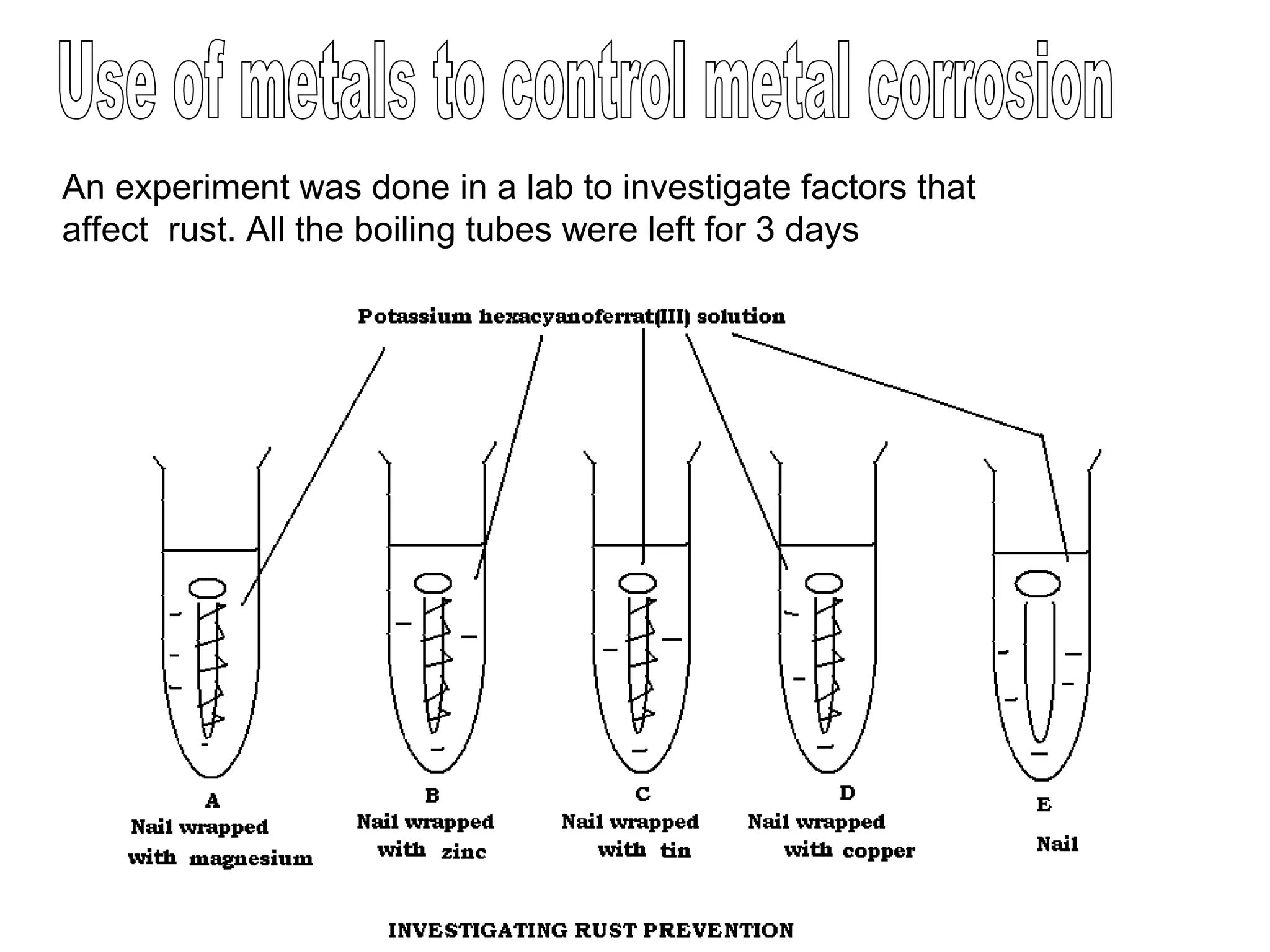
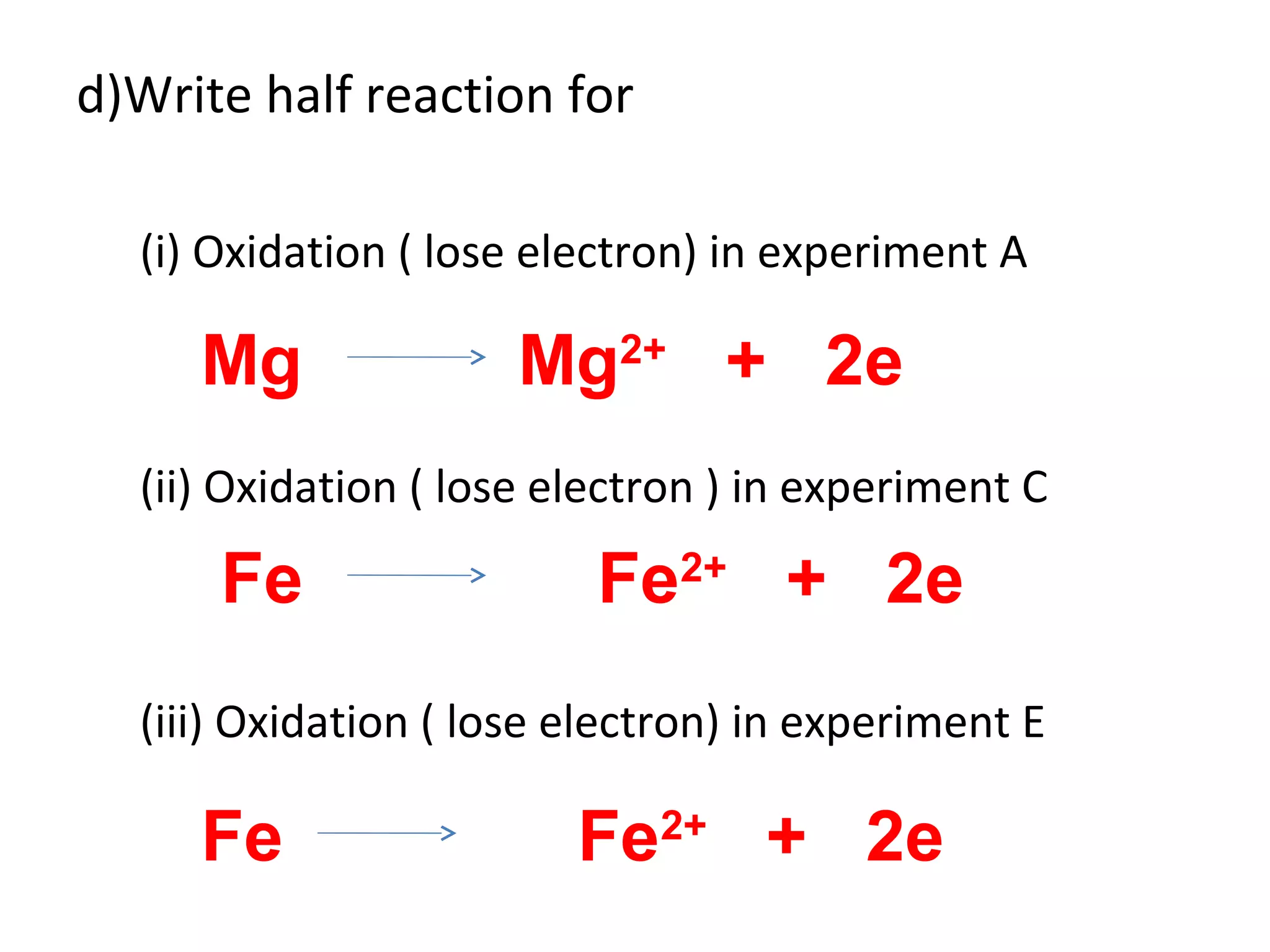
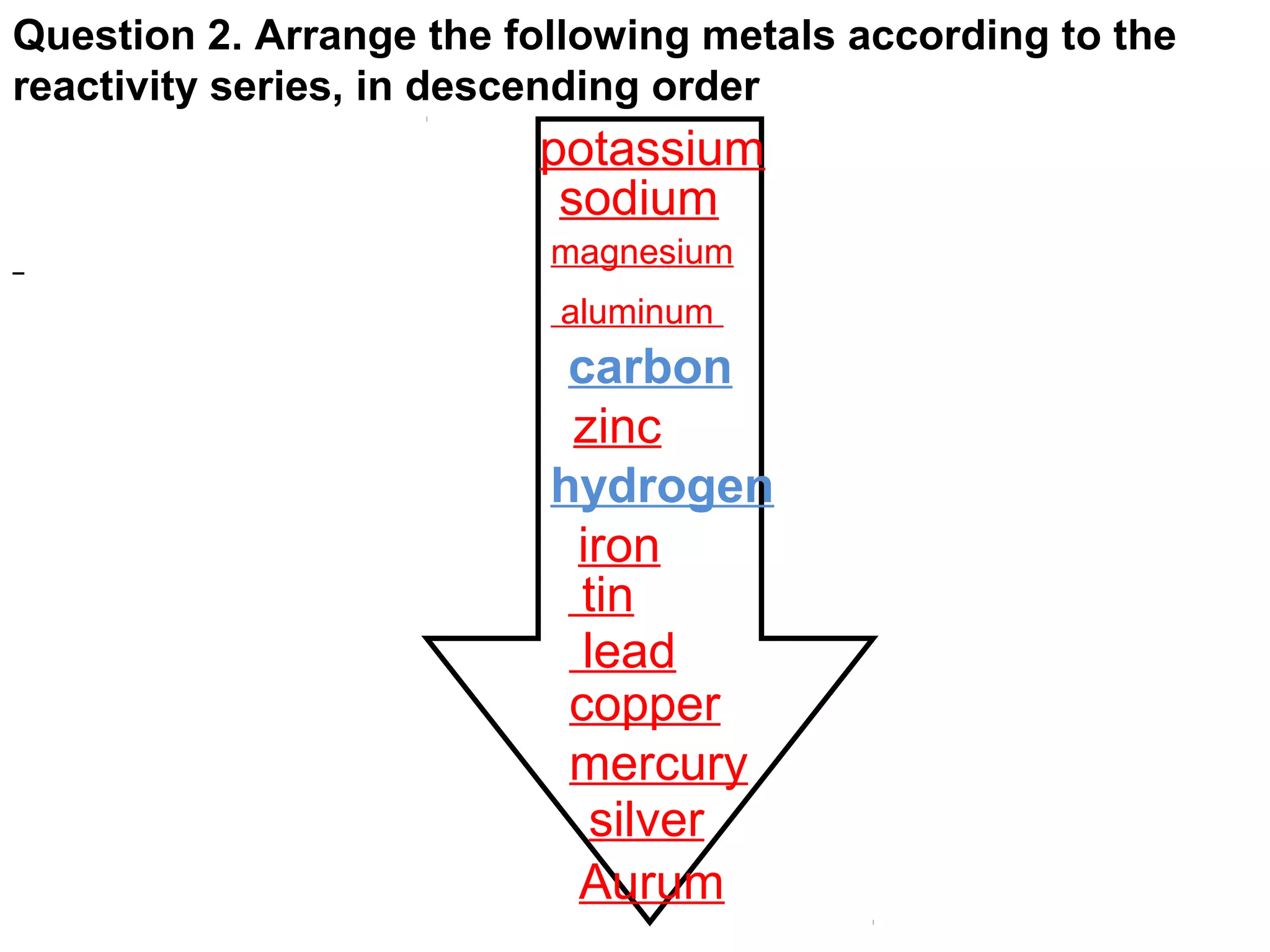
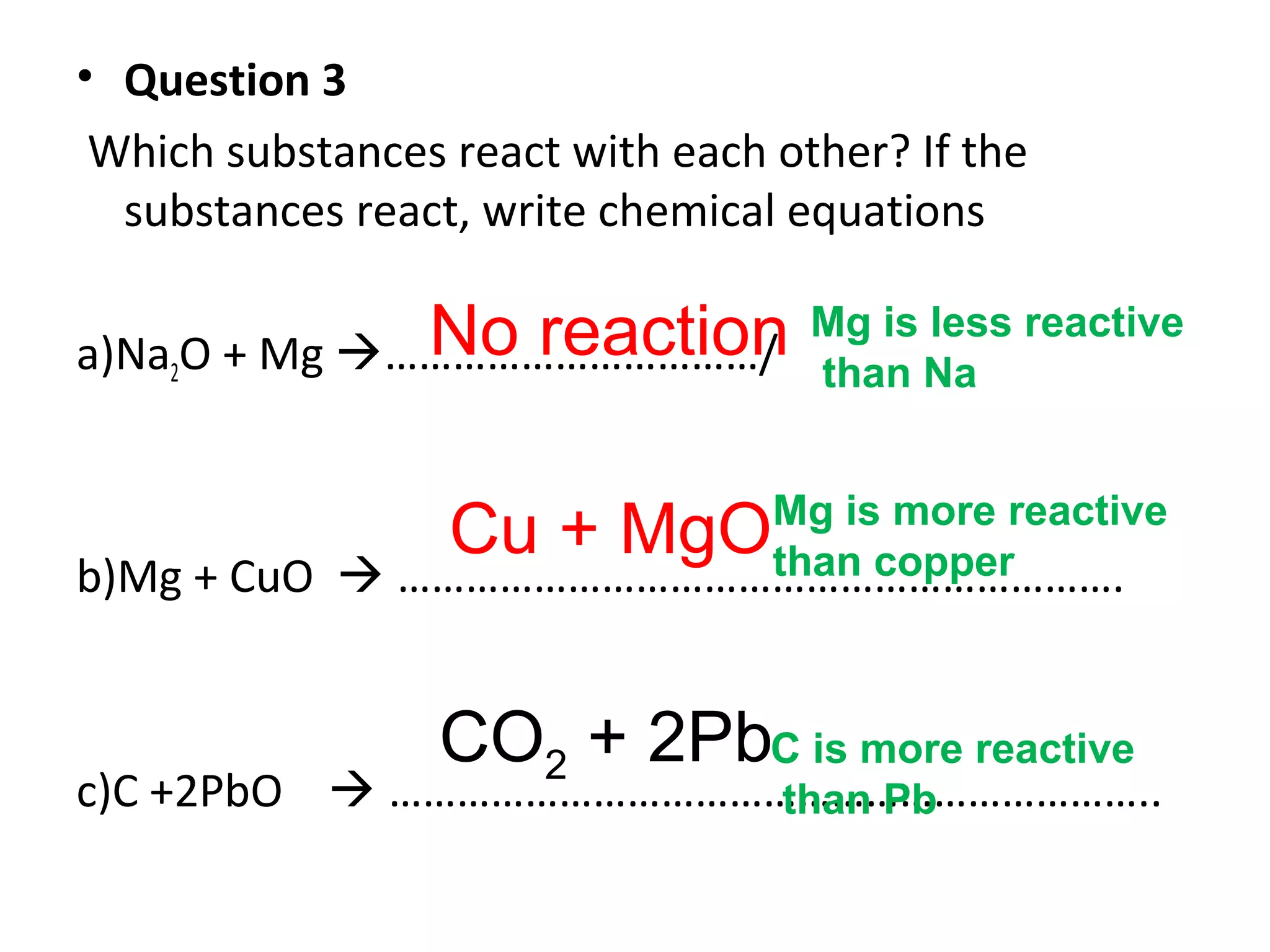
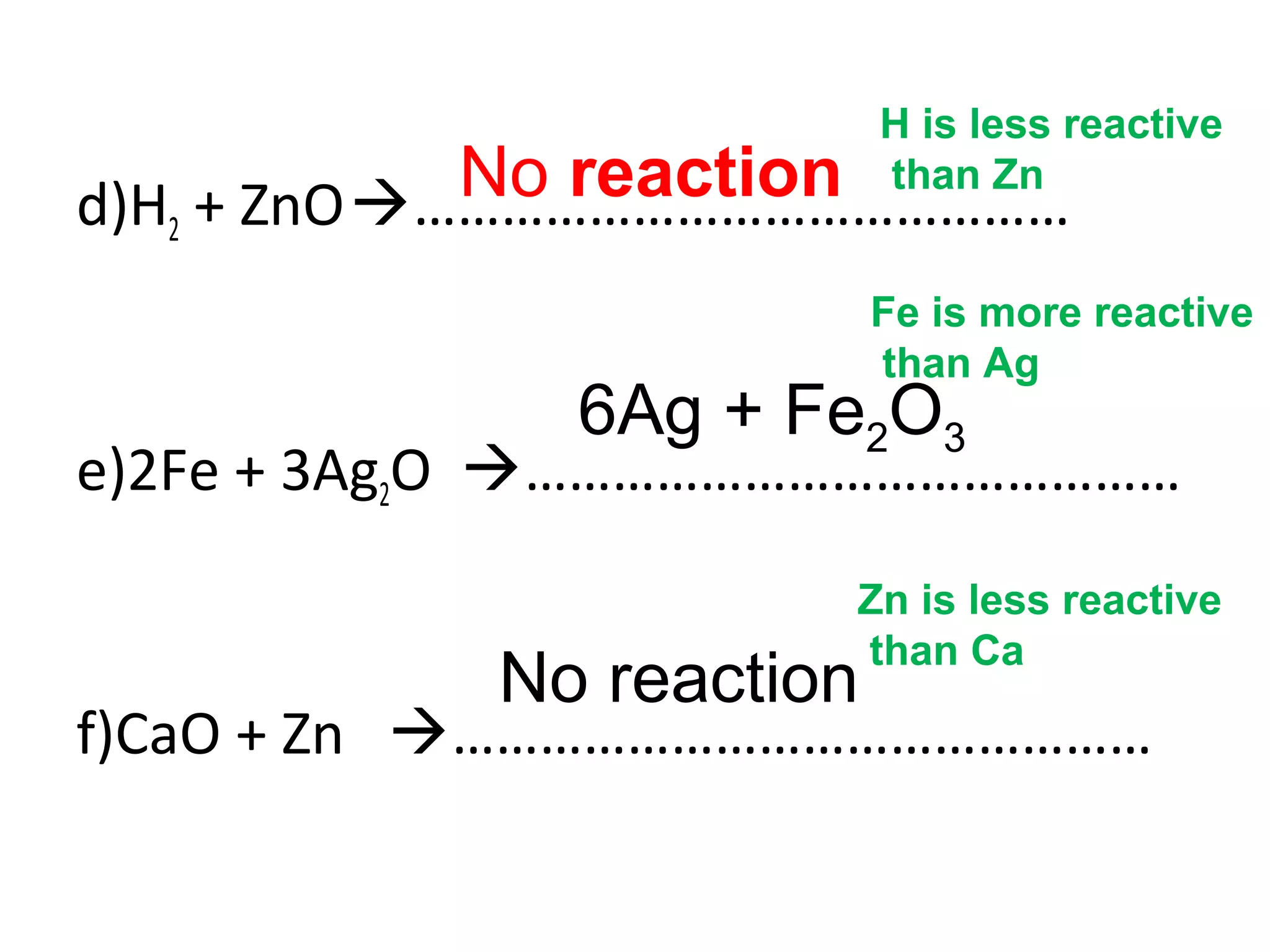
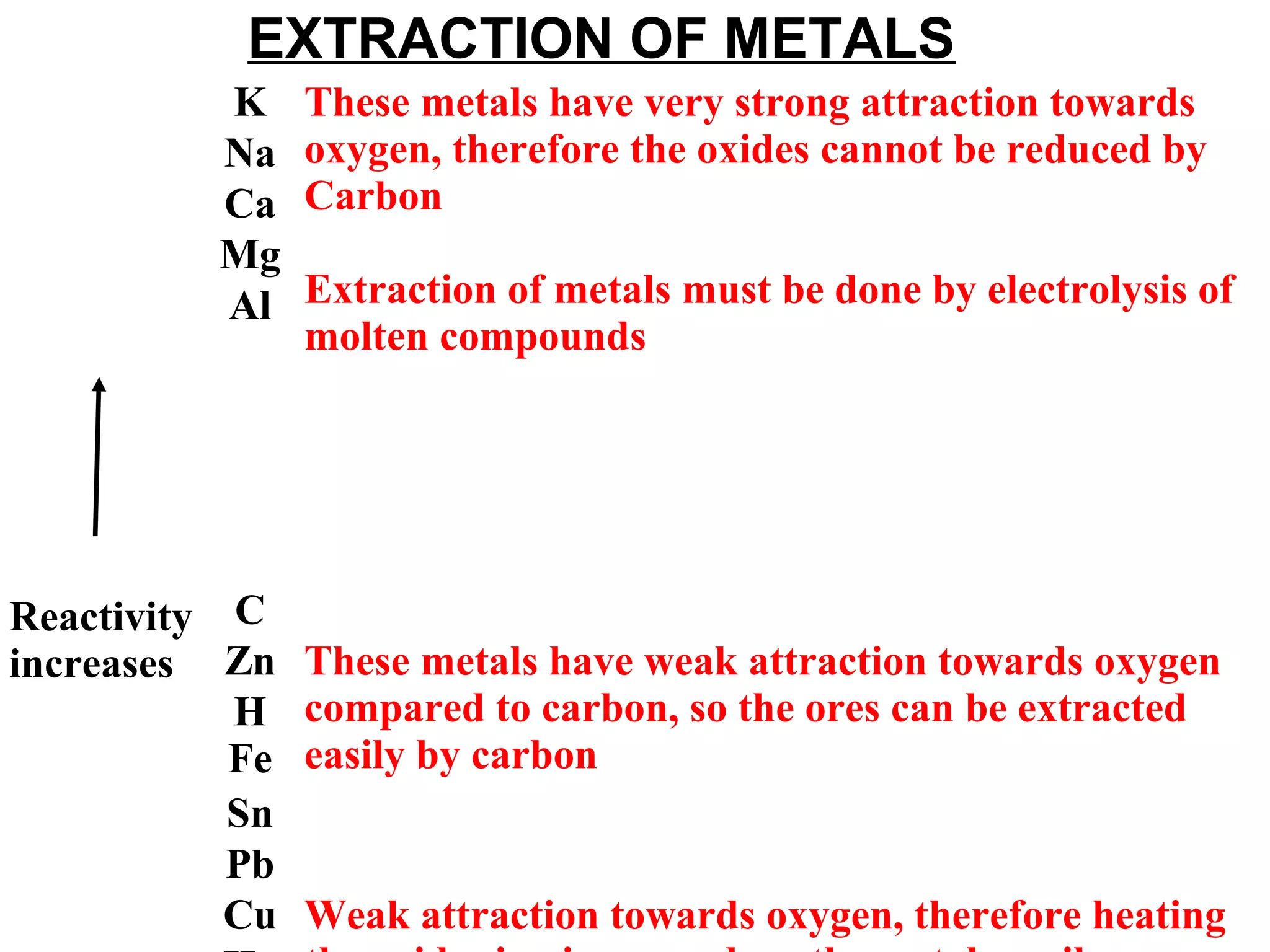
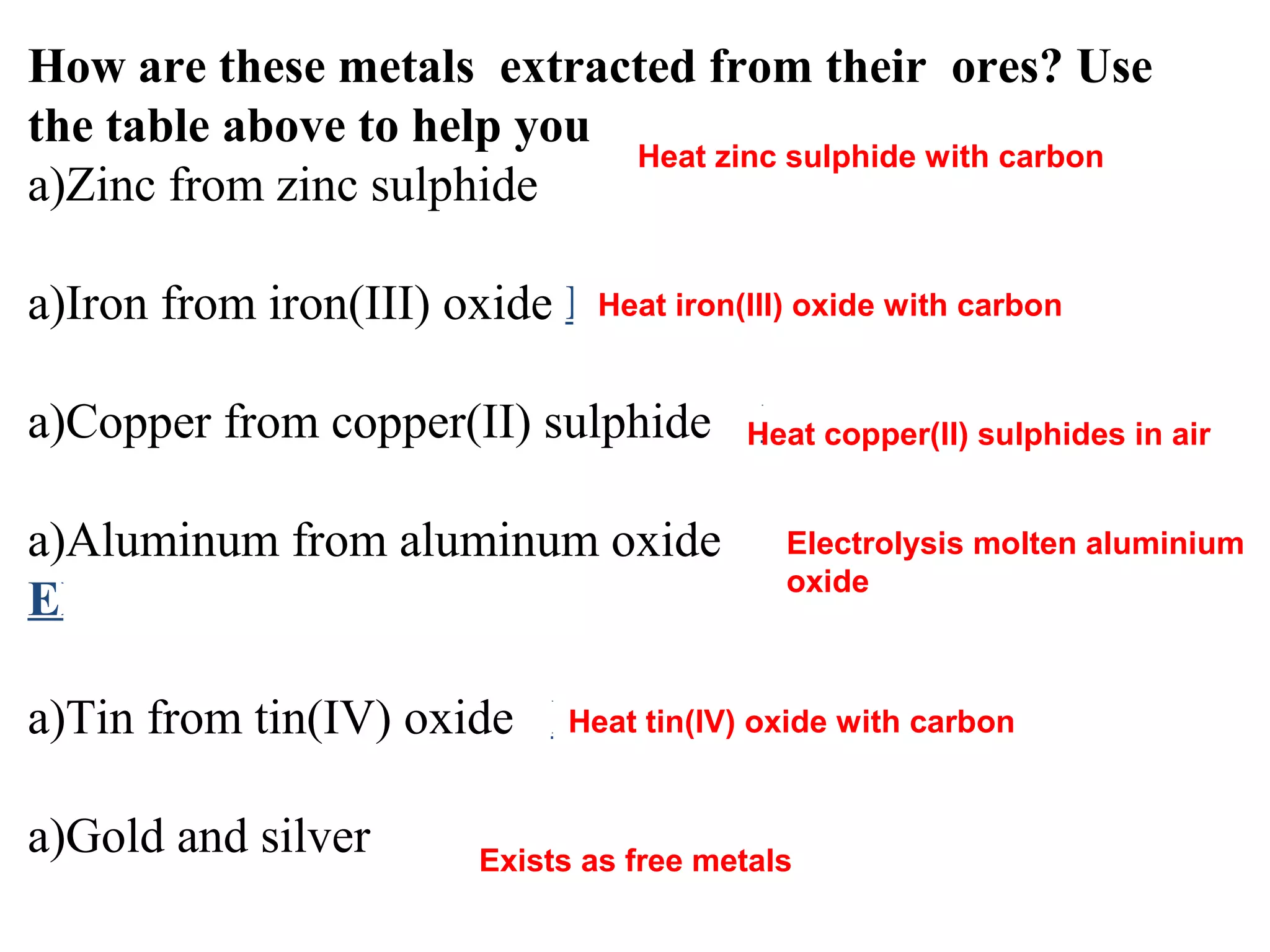
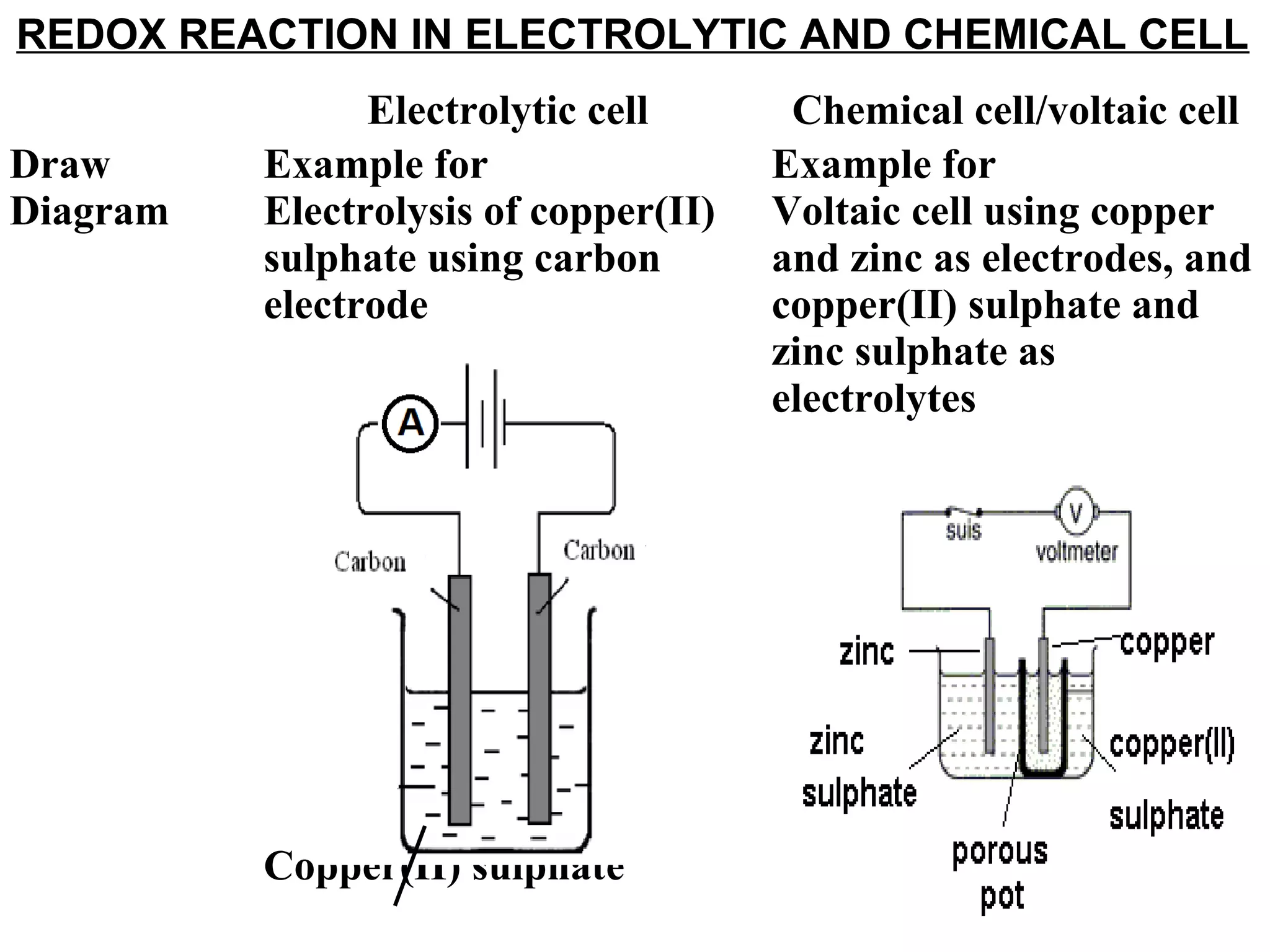
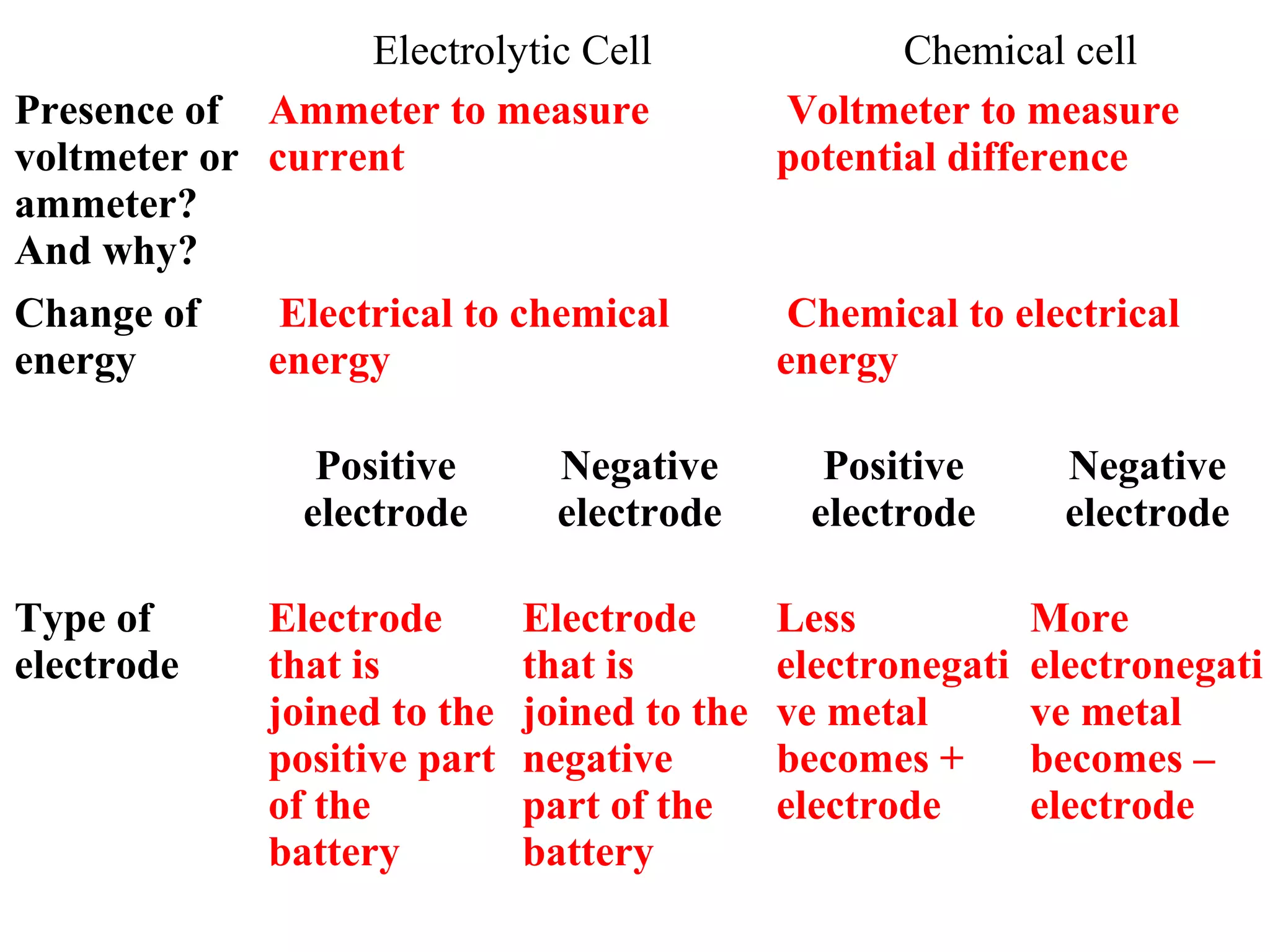
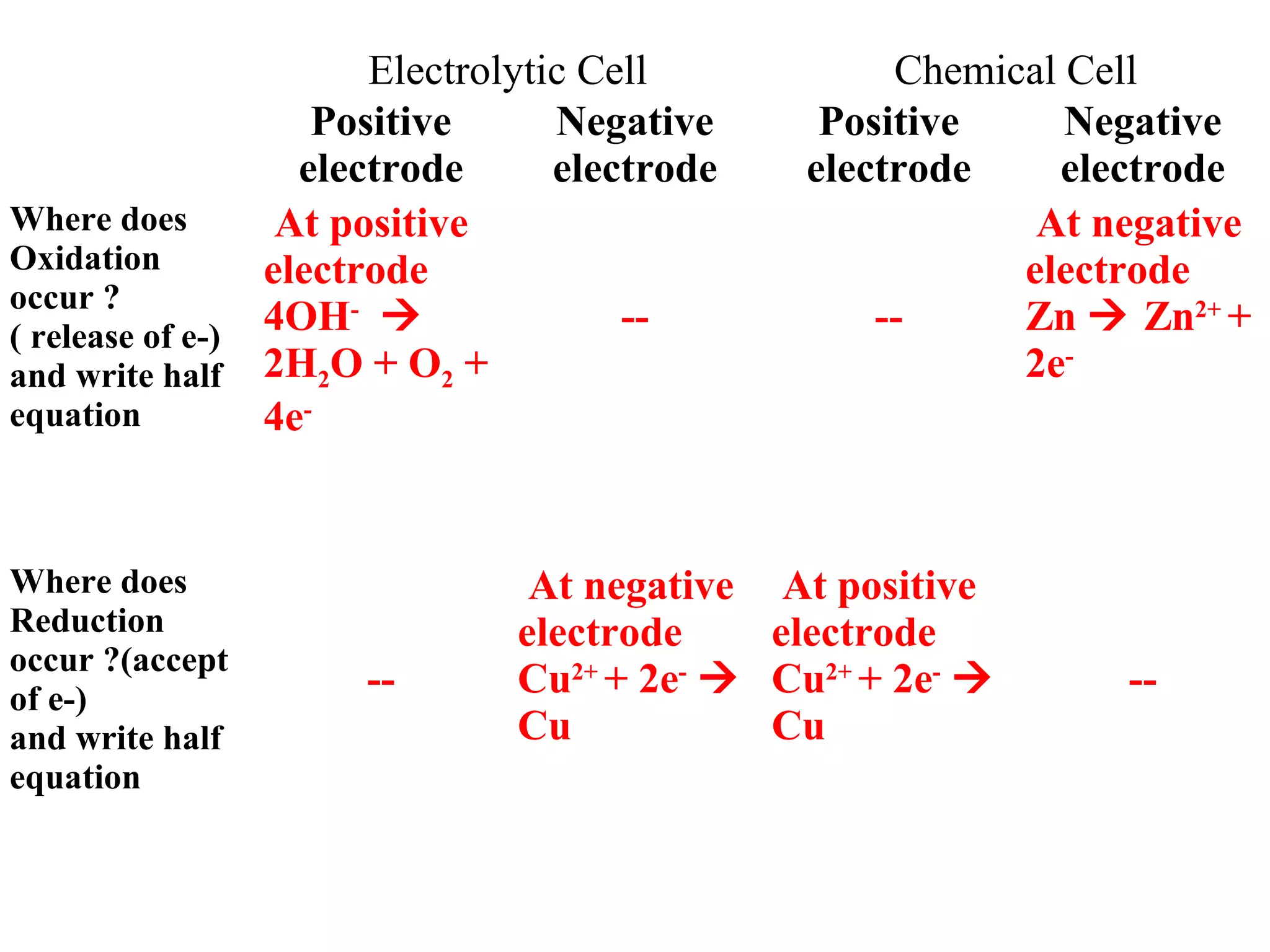
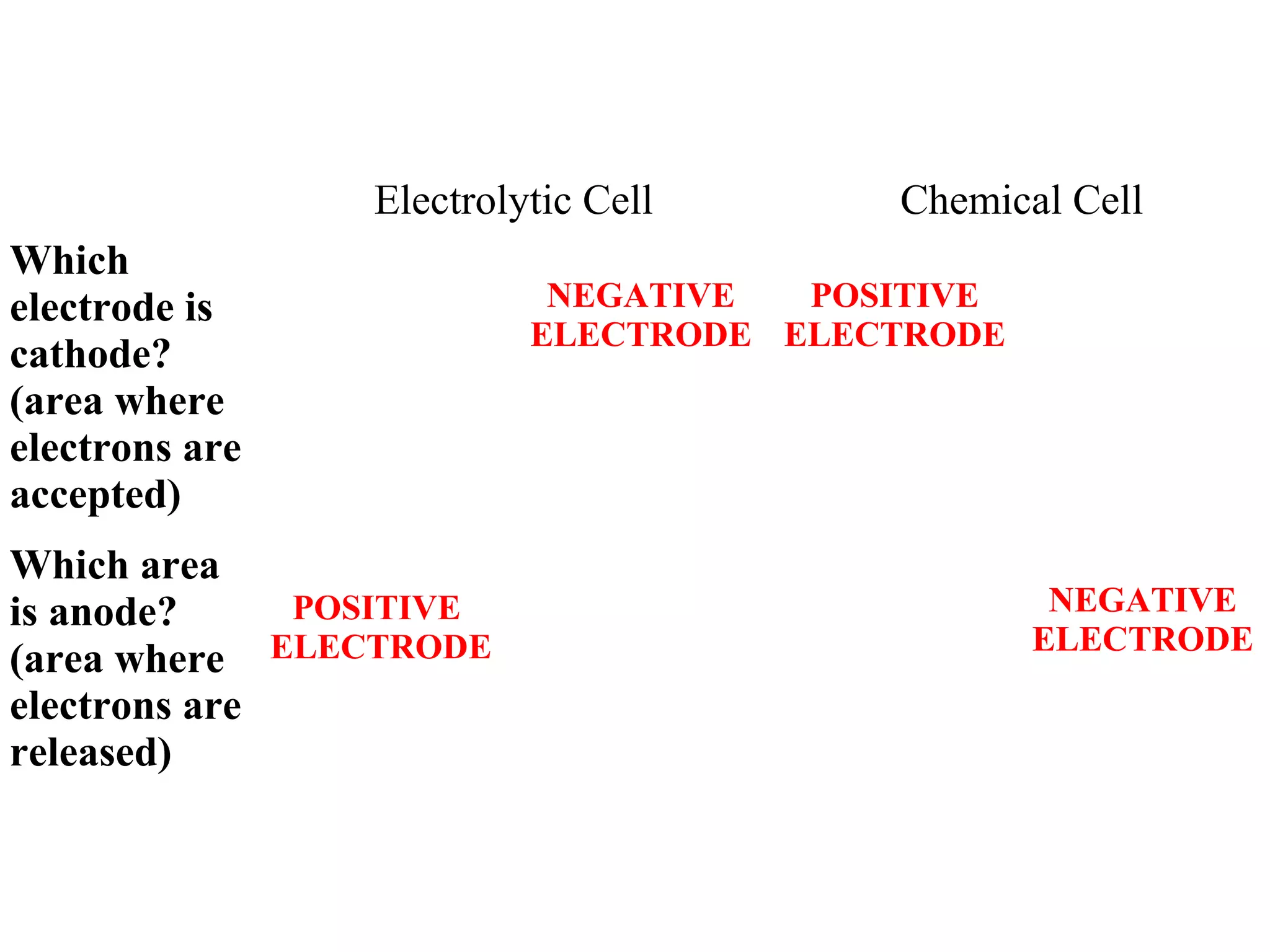
The document discusses the process of rusting, highlighting the chemical reactions involving iron, water, and oxygen that lead to the formation of rust (hydrated iron (III) oxide). It explains the electrochemical principles behind rusting, preventive measures such as protective coatings, and the reactivity series of metals that influence corrosion resistance. Additionally, it includes experimental observations and theoretical explanations for the rusting process and methods of metal extraction.