Embed presentation
Downloaded 26 times

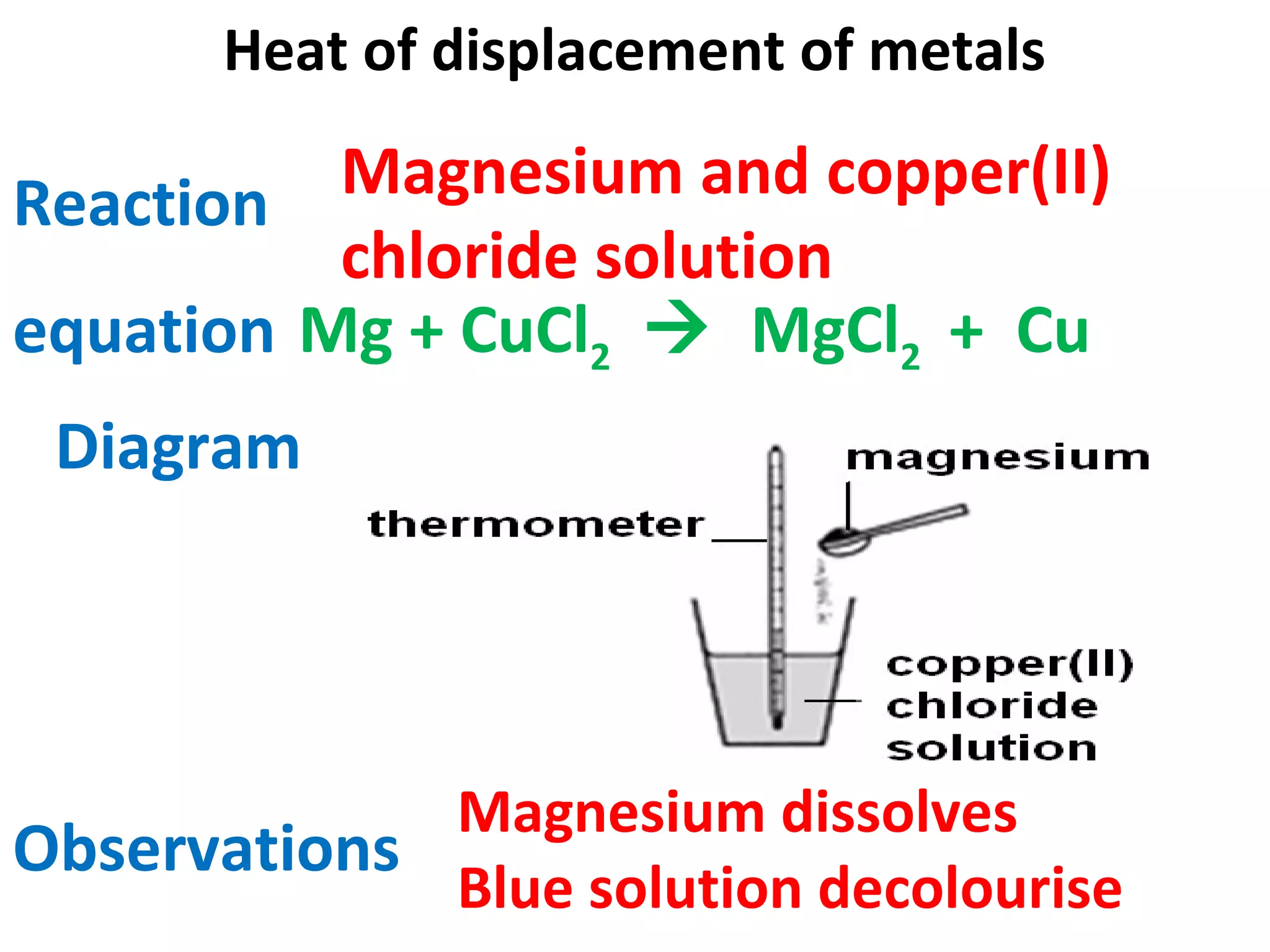
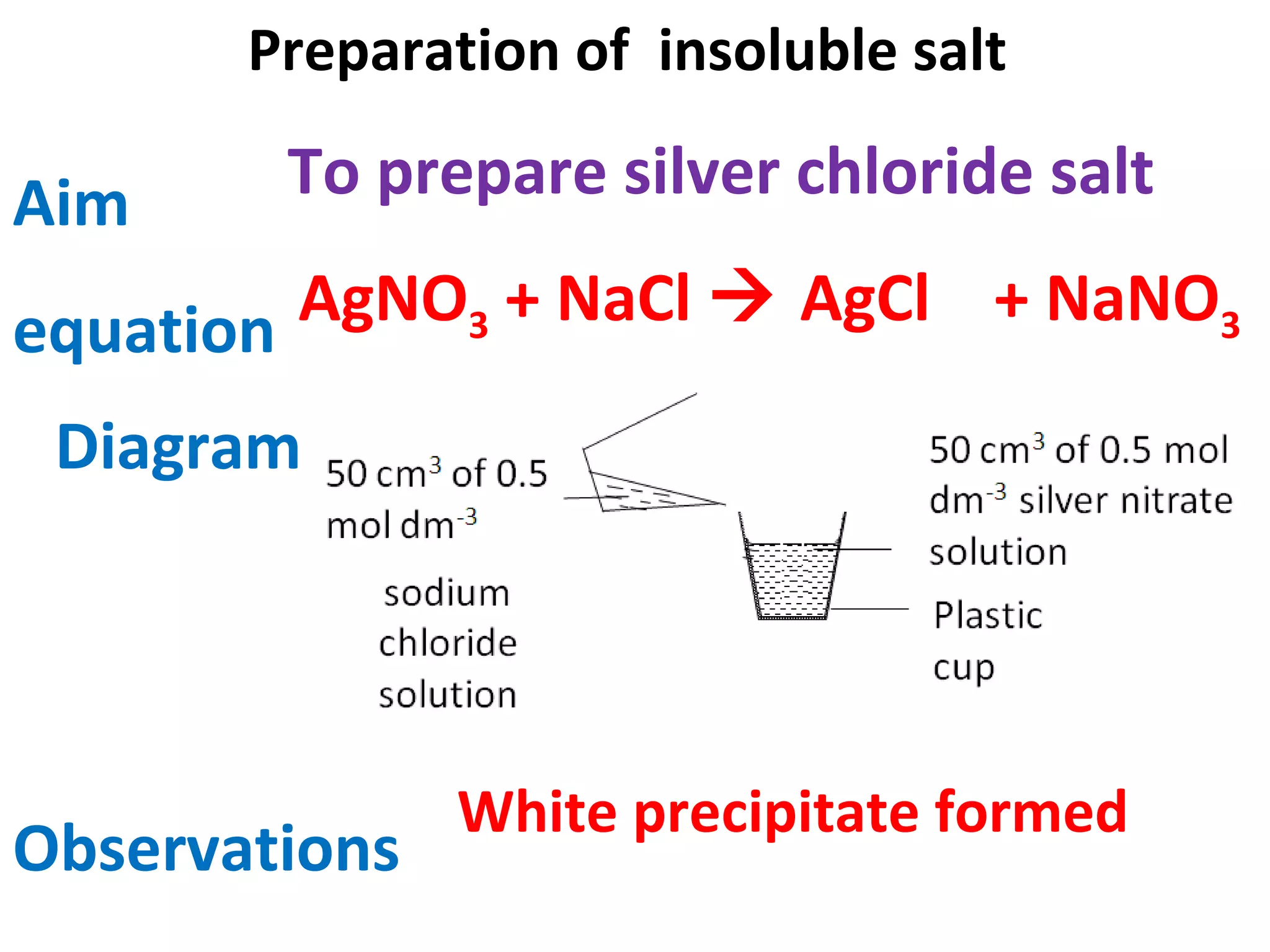
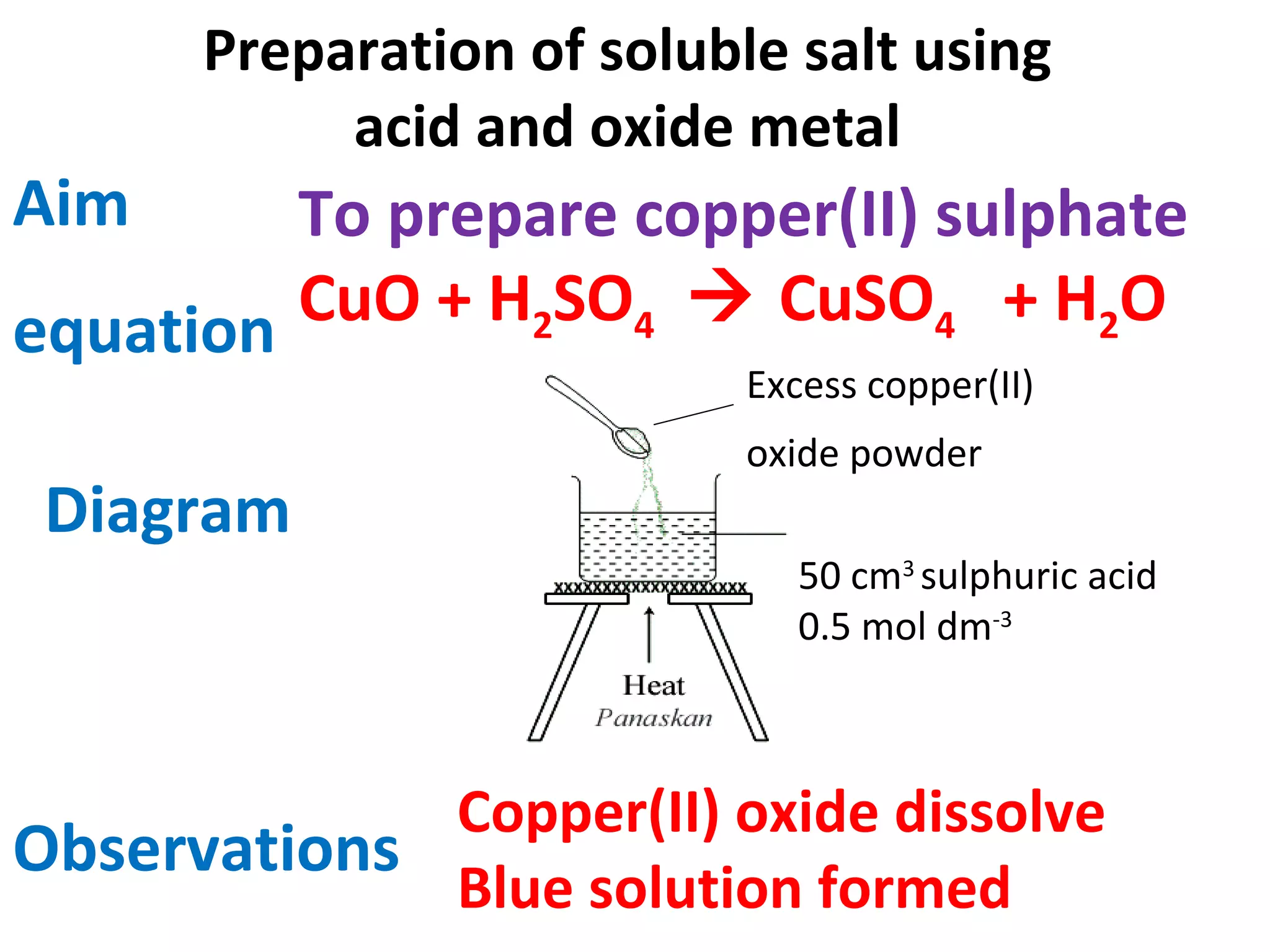
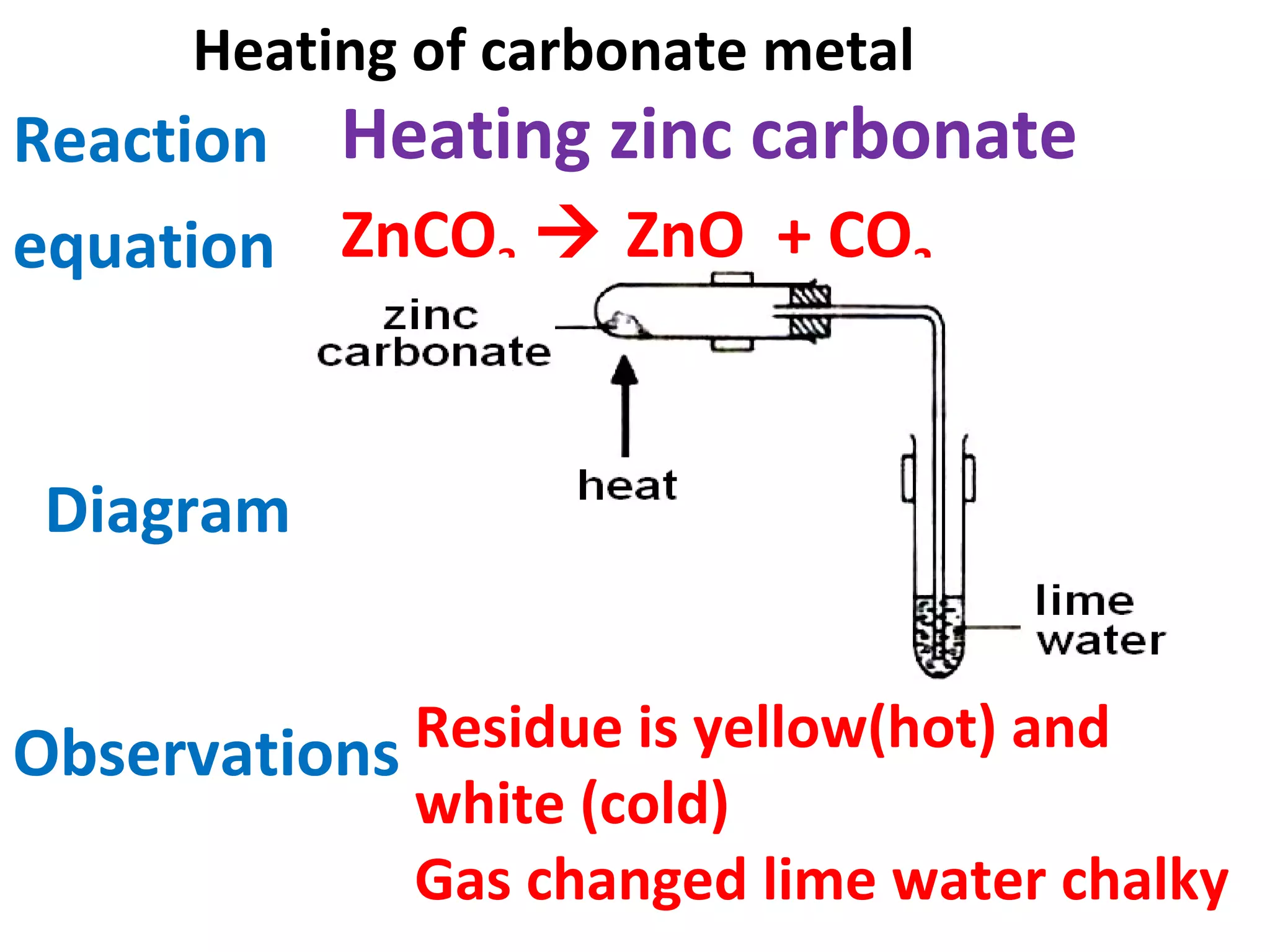
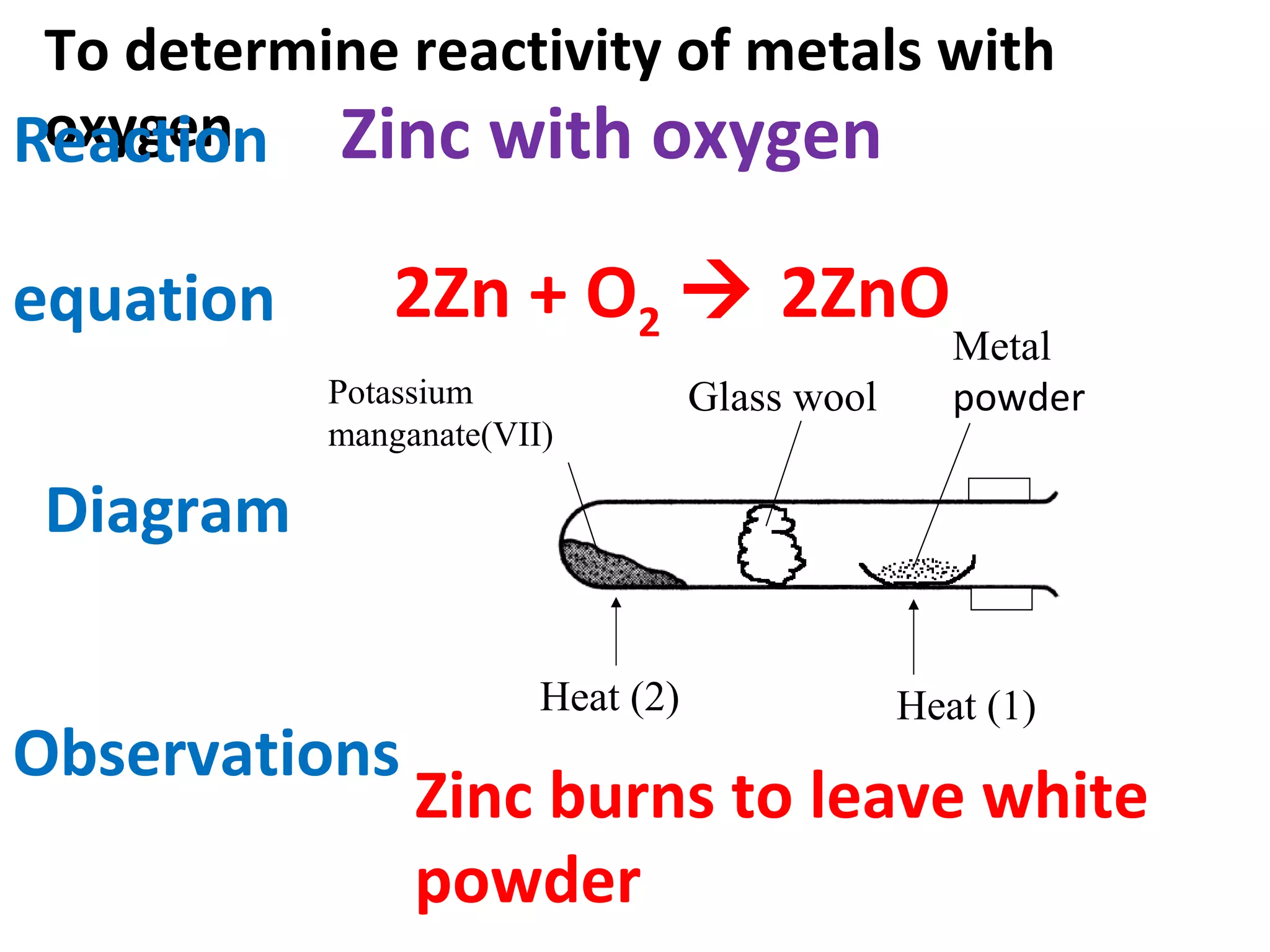
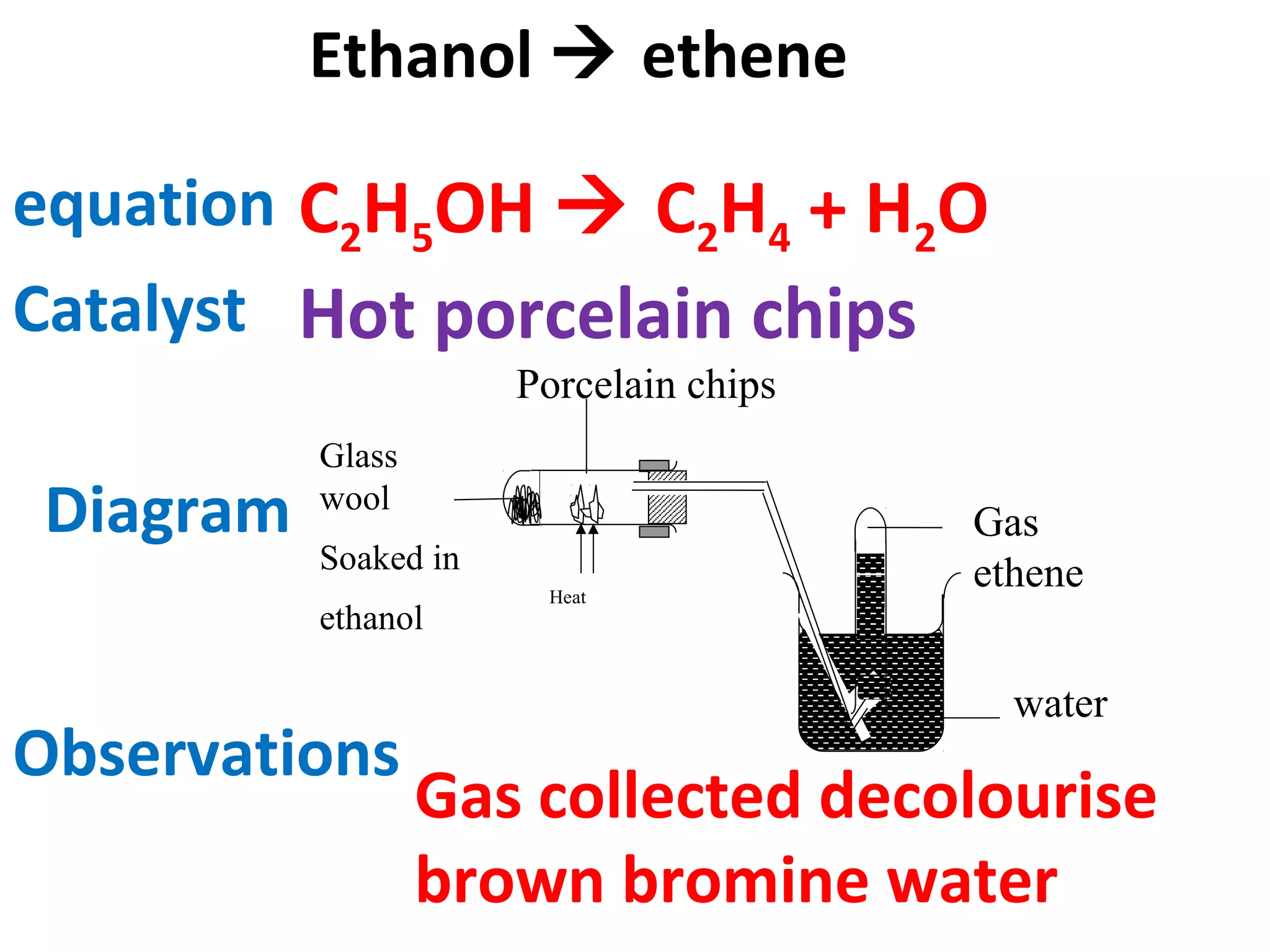


This document contains descriptions and diagrams of several chemical reactions and experiments: 1) A reaction between magnesium and copper chloride produces magnesium chloride and copper. 2) Precipitation of silver chloride is achieved through a reaction of silver nitrate and sodium chloride. 3) Copper(II) oxide reacts with sulfuric acid to produce copper(II) sulfate and water. 4) Heating zinc carbonate produces zinc oxide and carbon dioxide, and the gas changes limewater to chalky.







