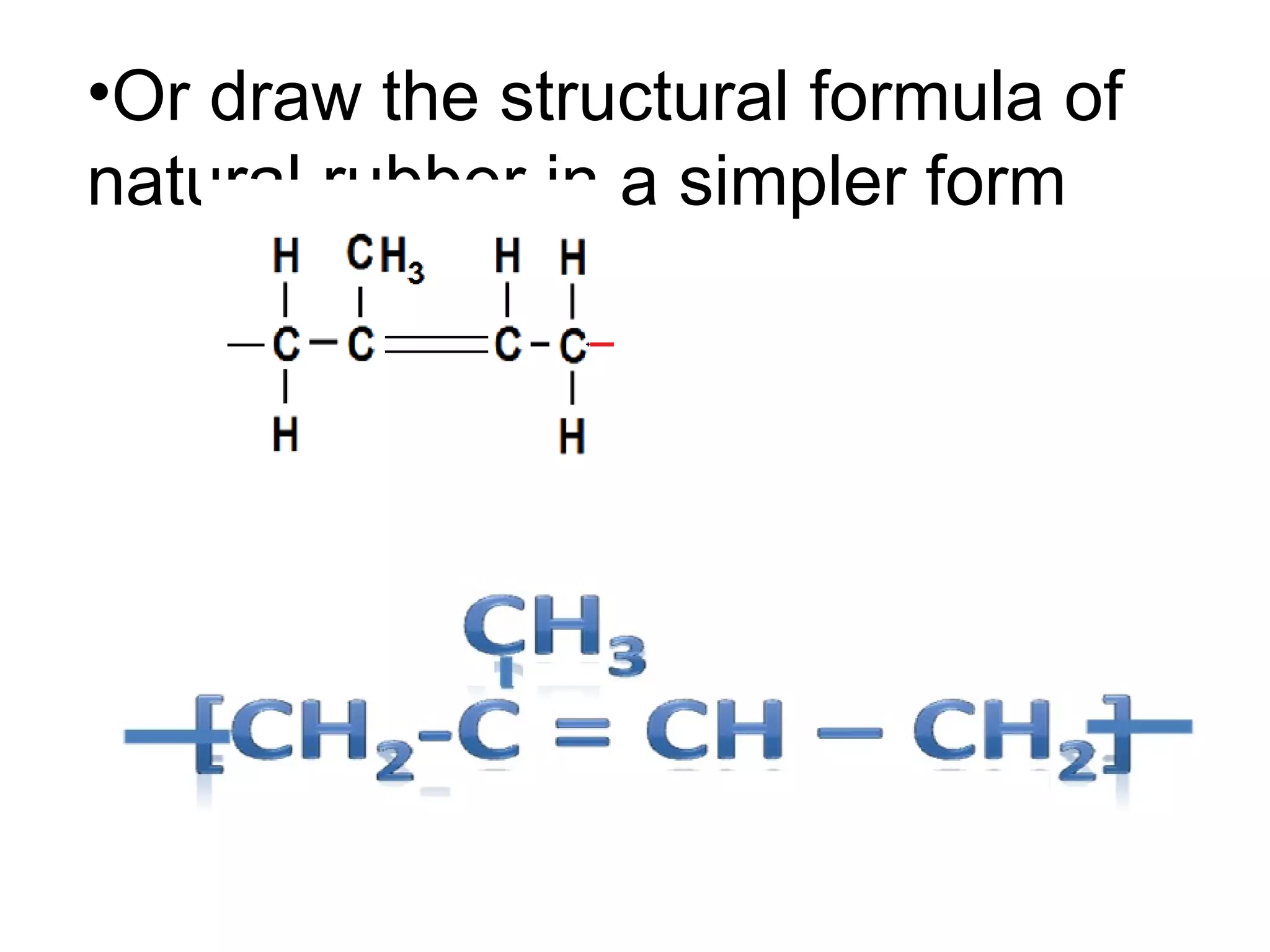
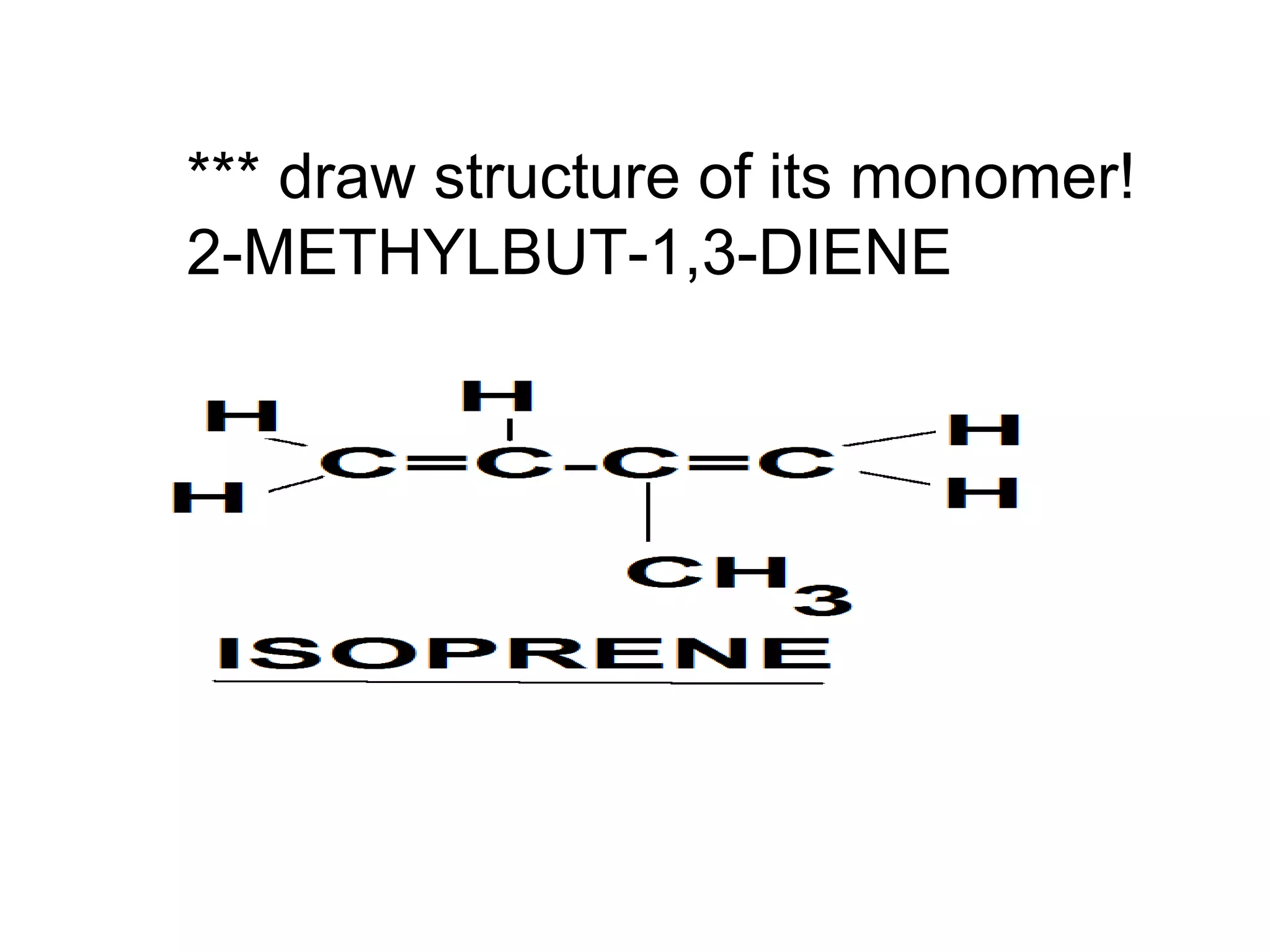
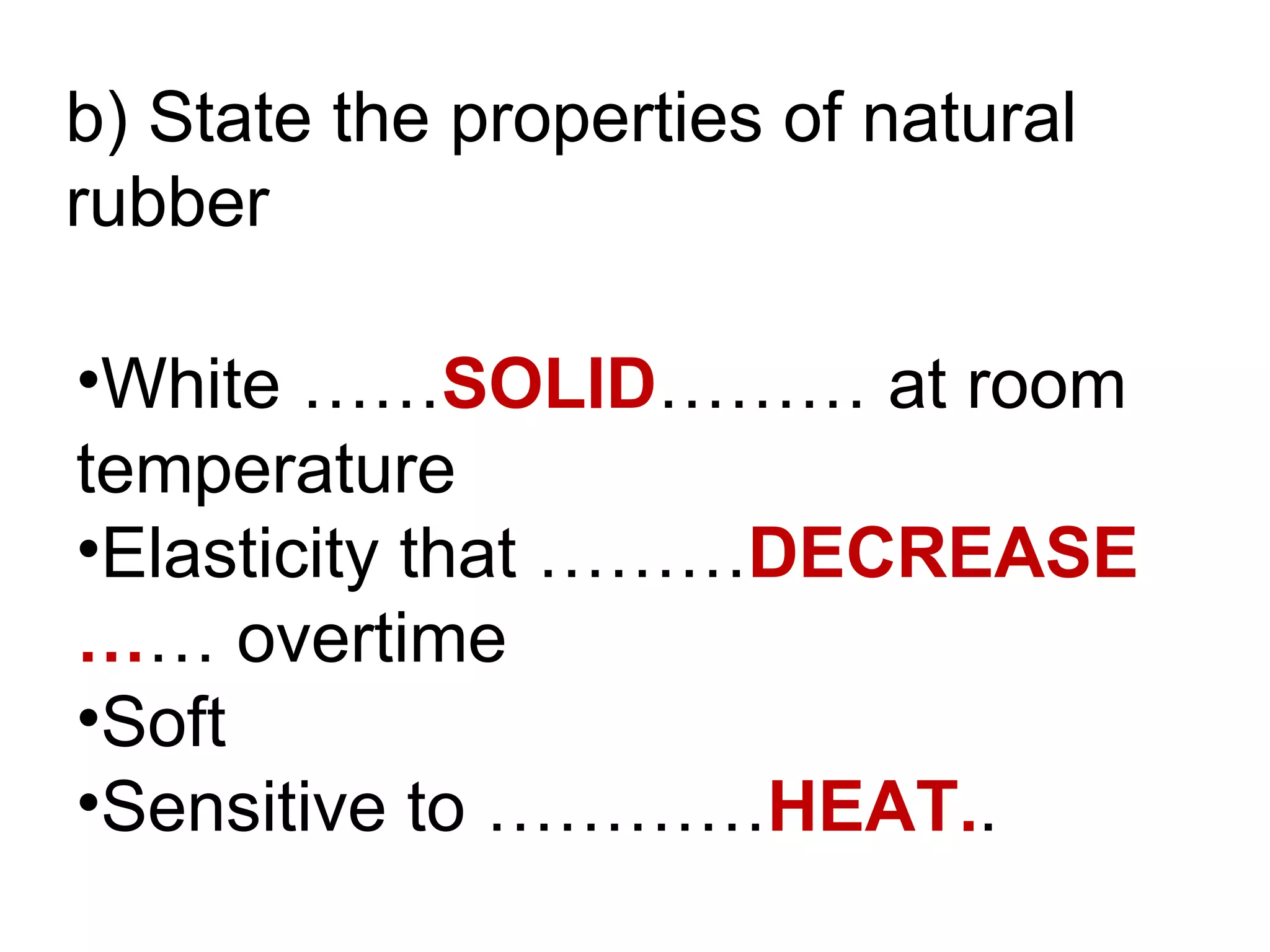
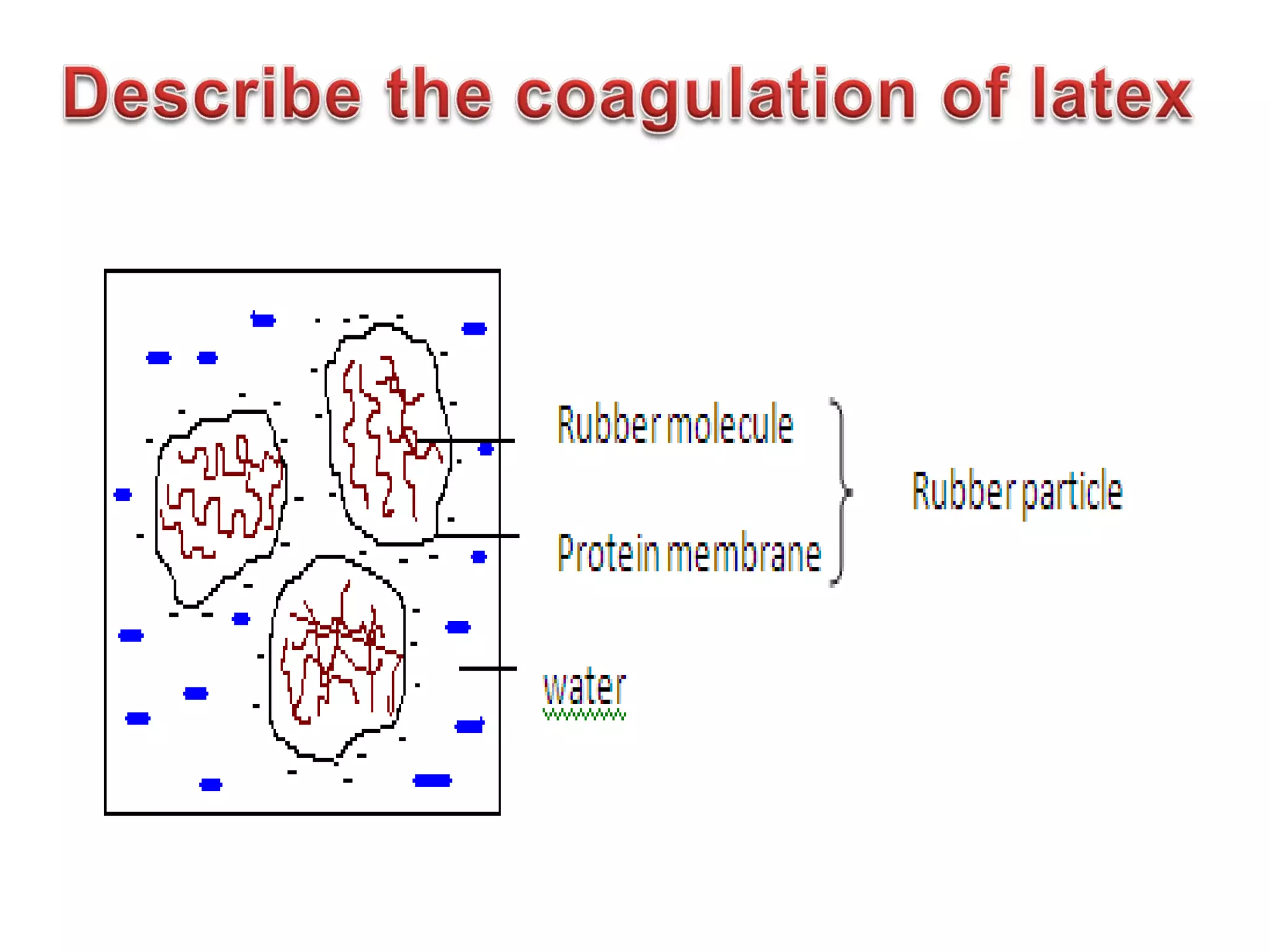
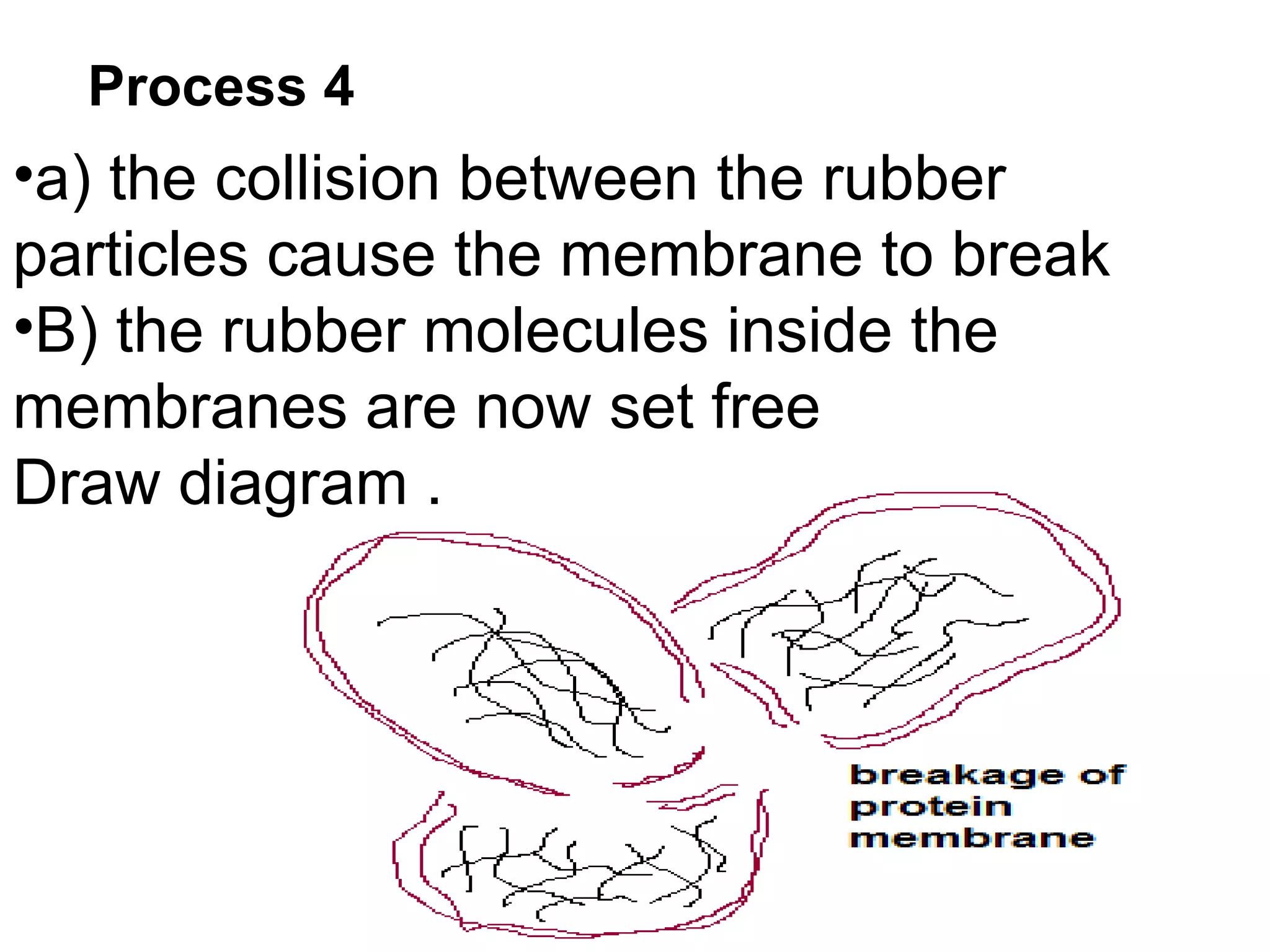
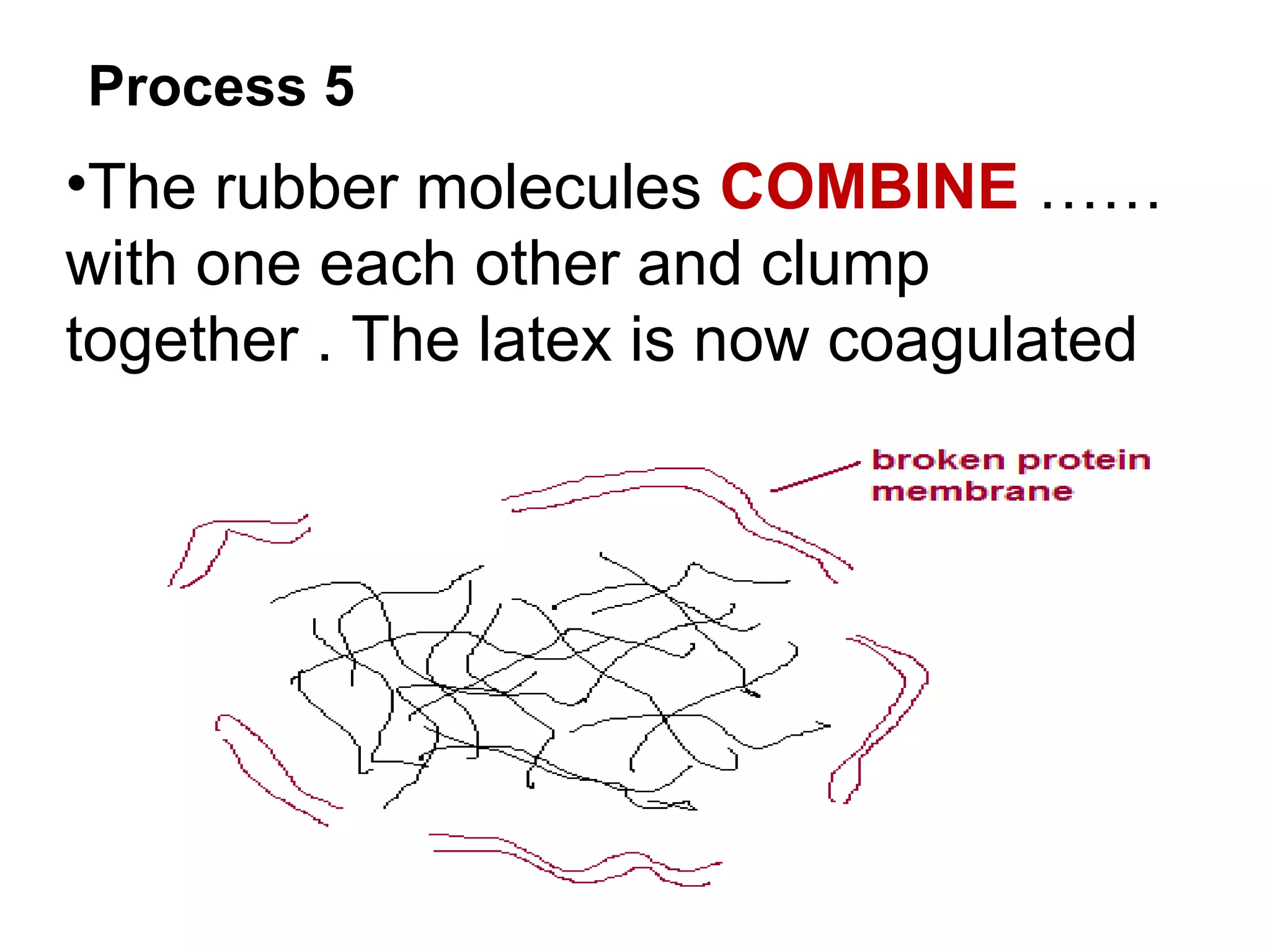
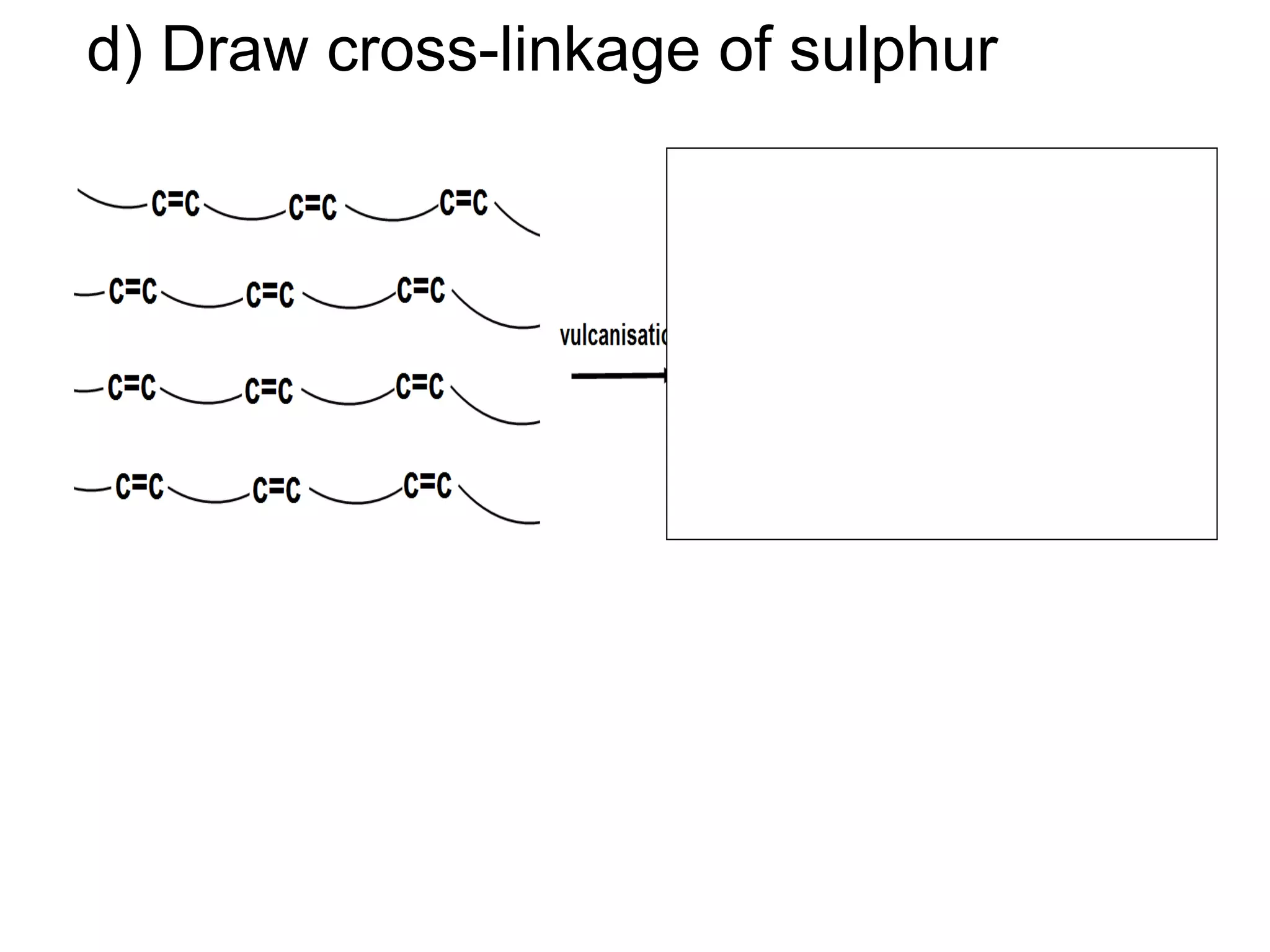
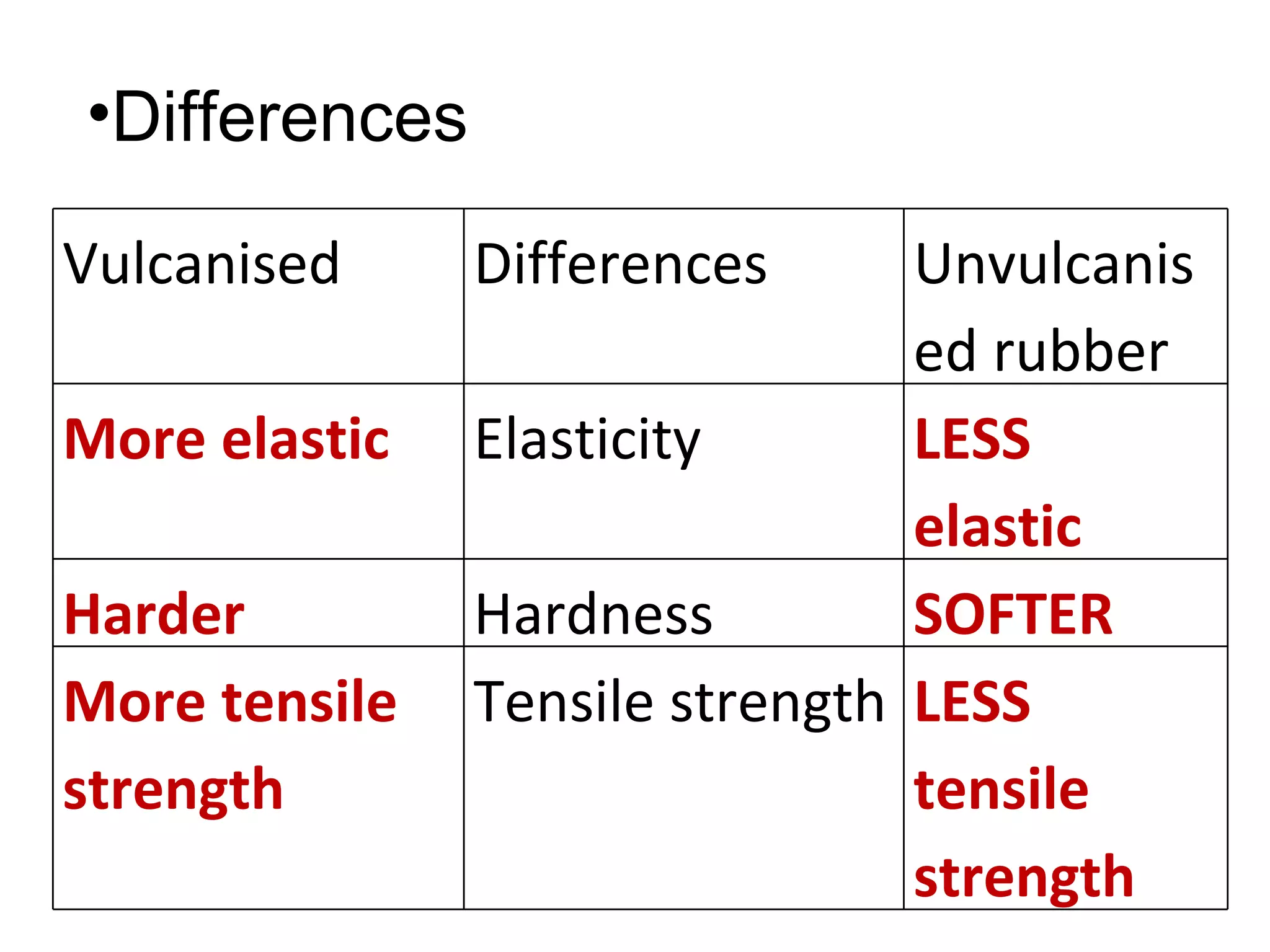
The document discusses key concepts related to fats and oils including:
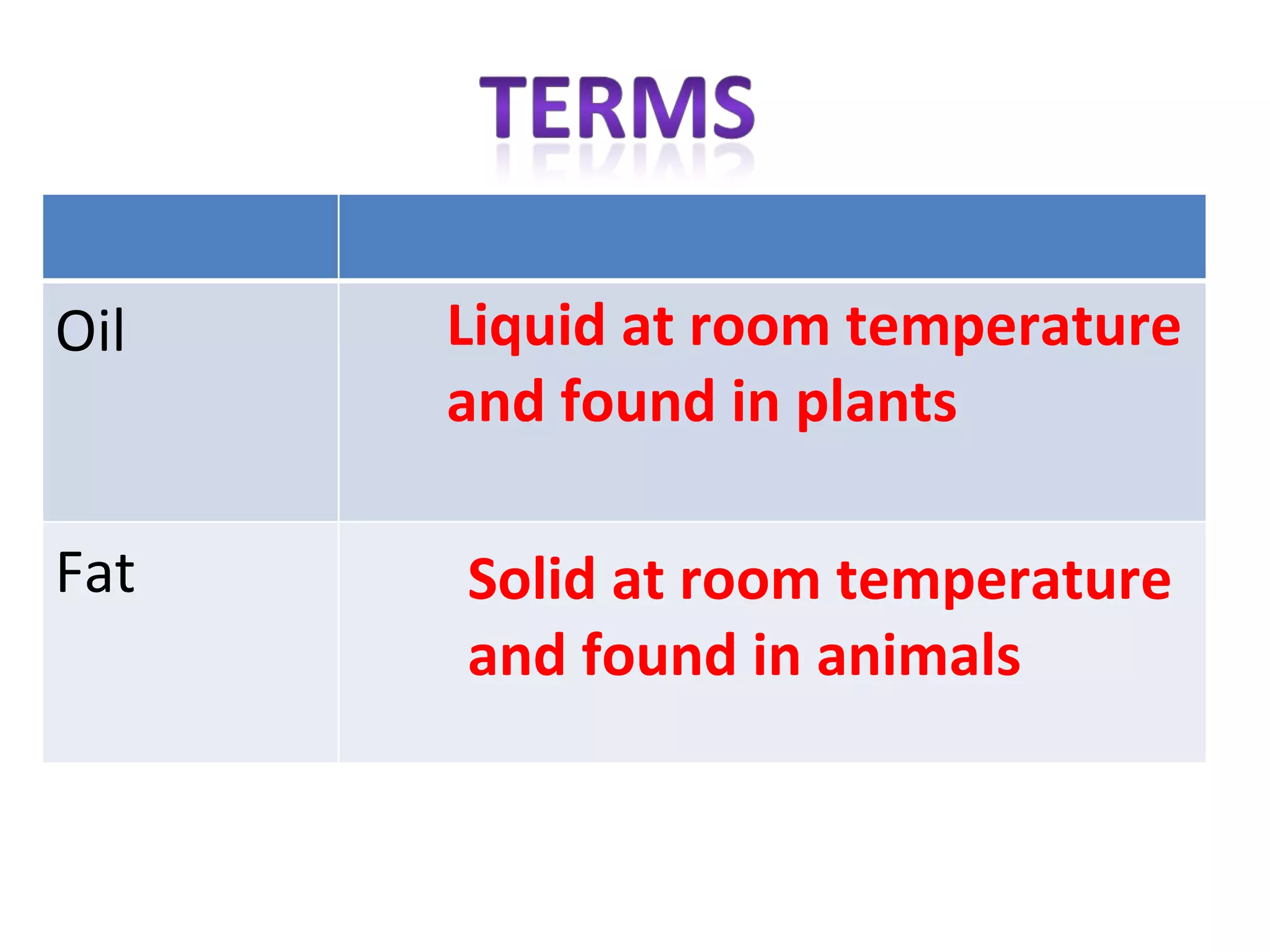
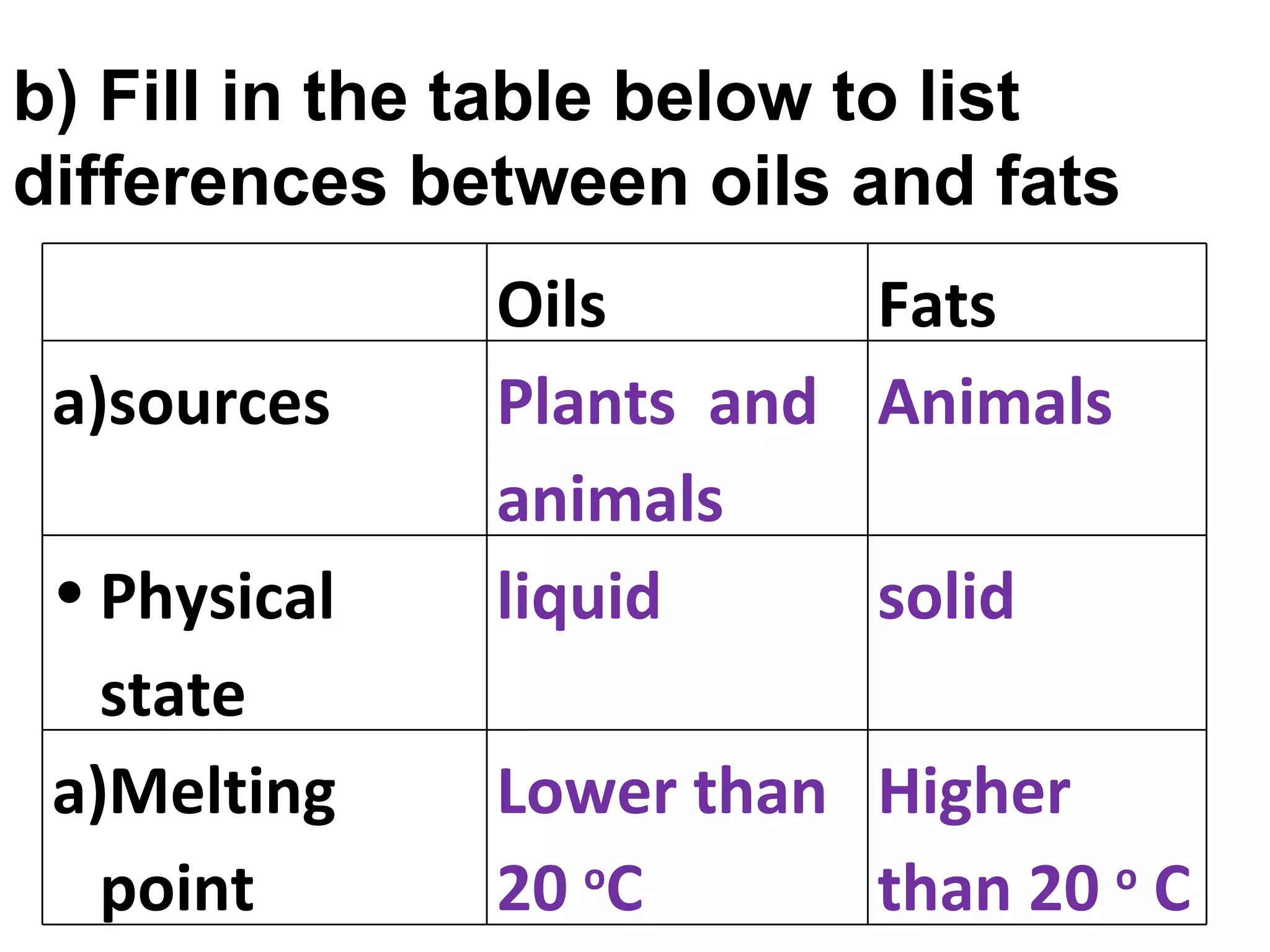
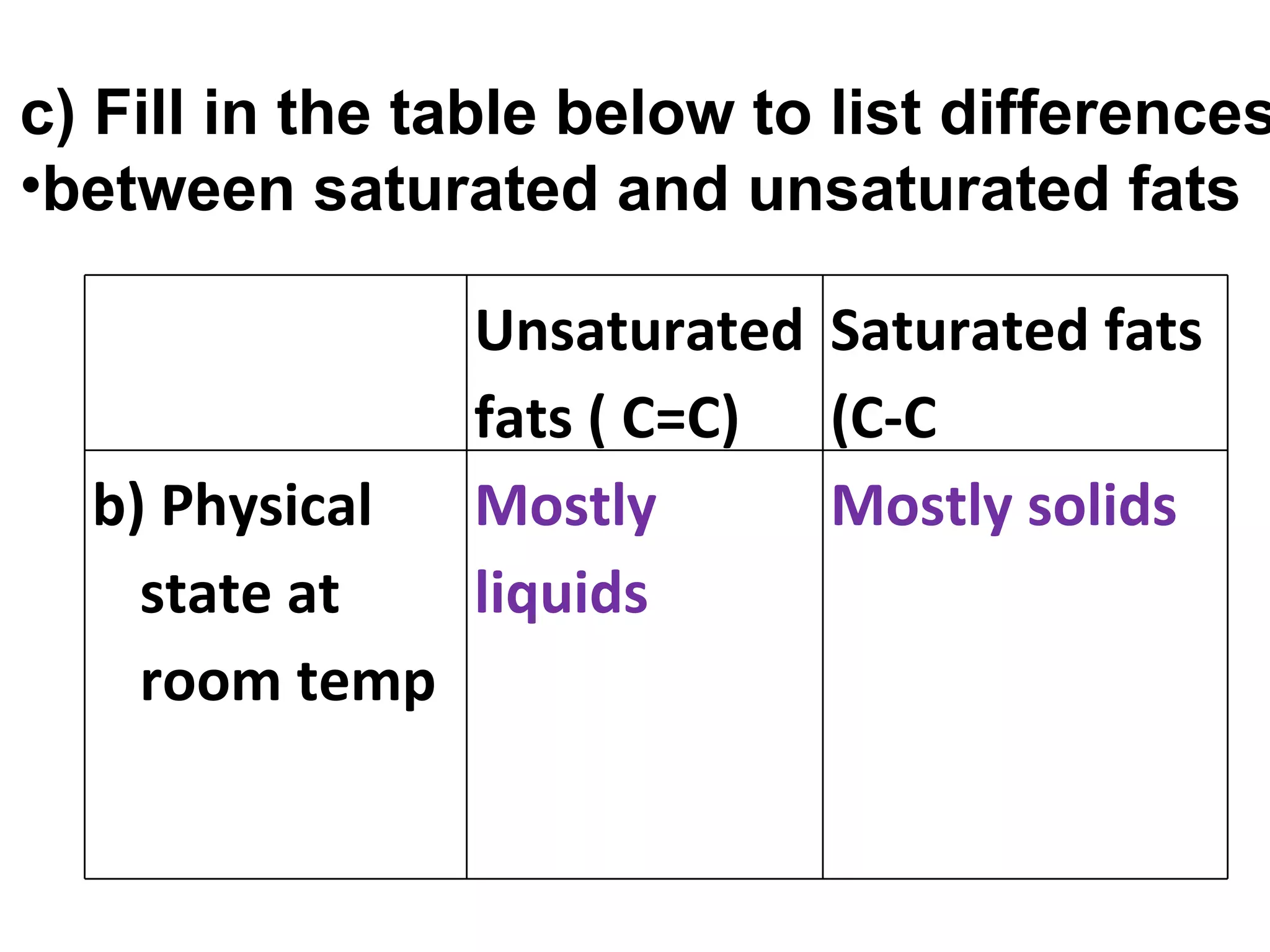
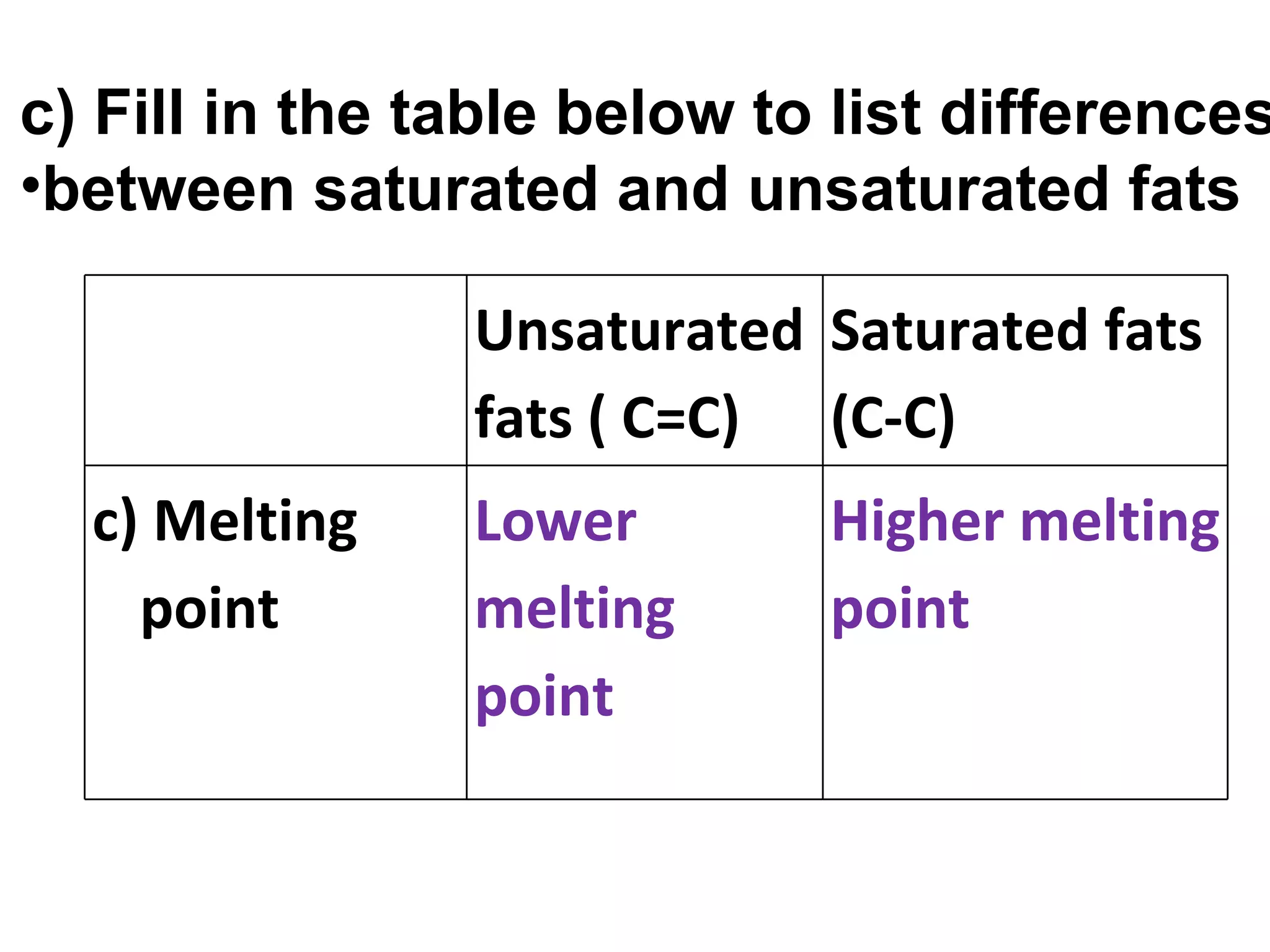
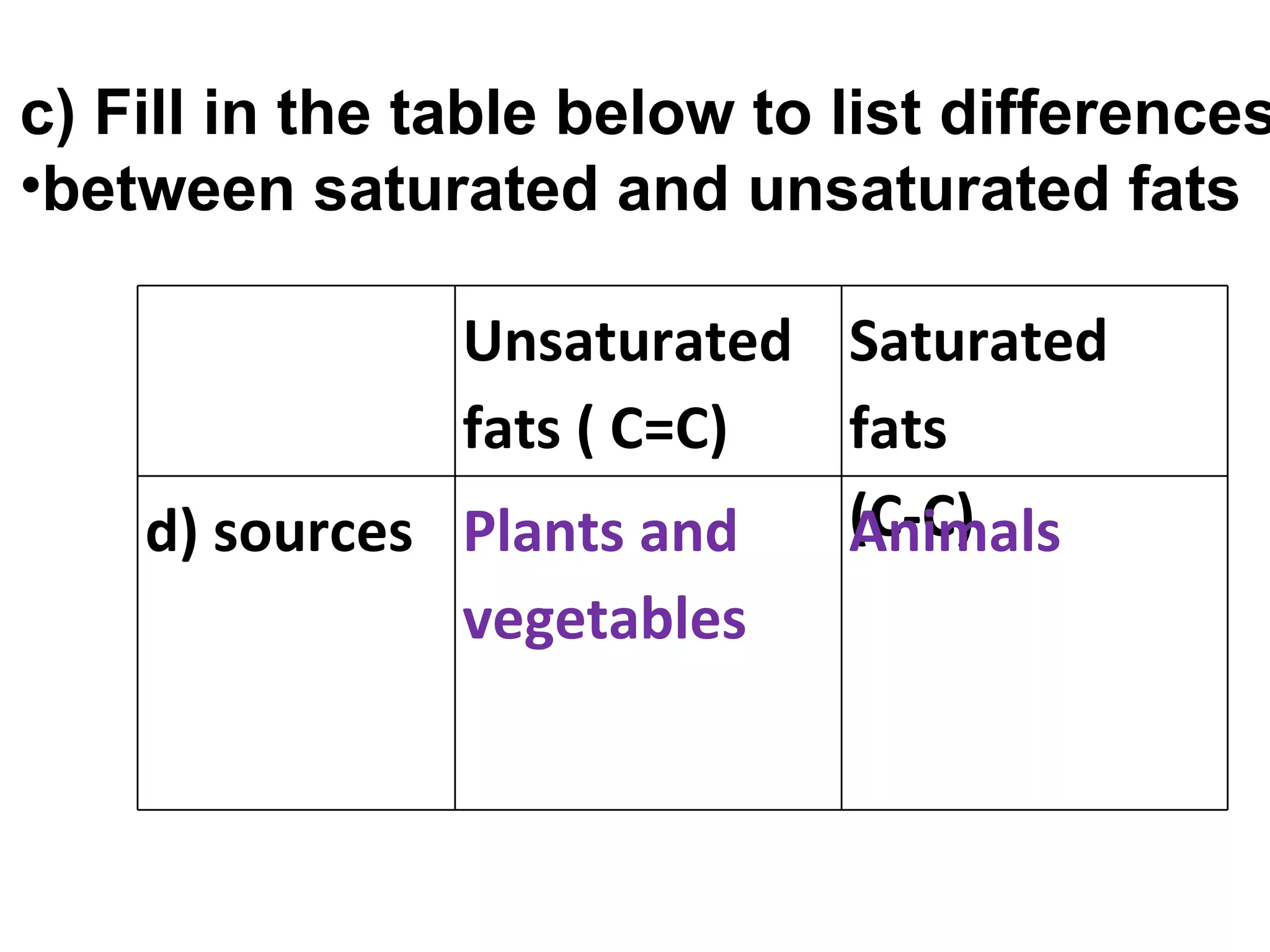
1) Fats are solid at room temperature and found in animals, while oils are liquid at room temperature and found in plants.
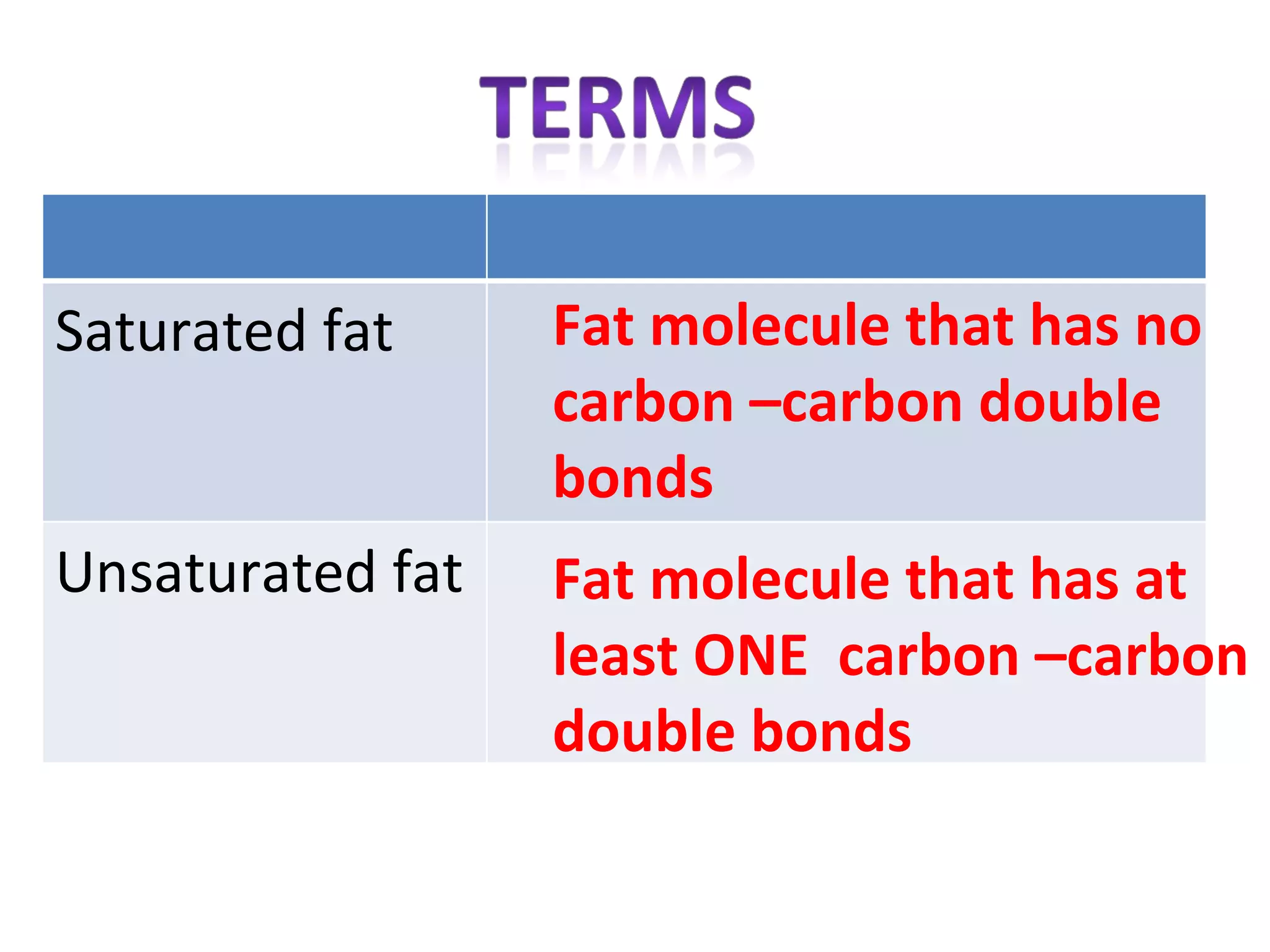
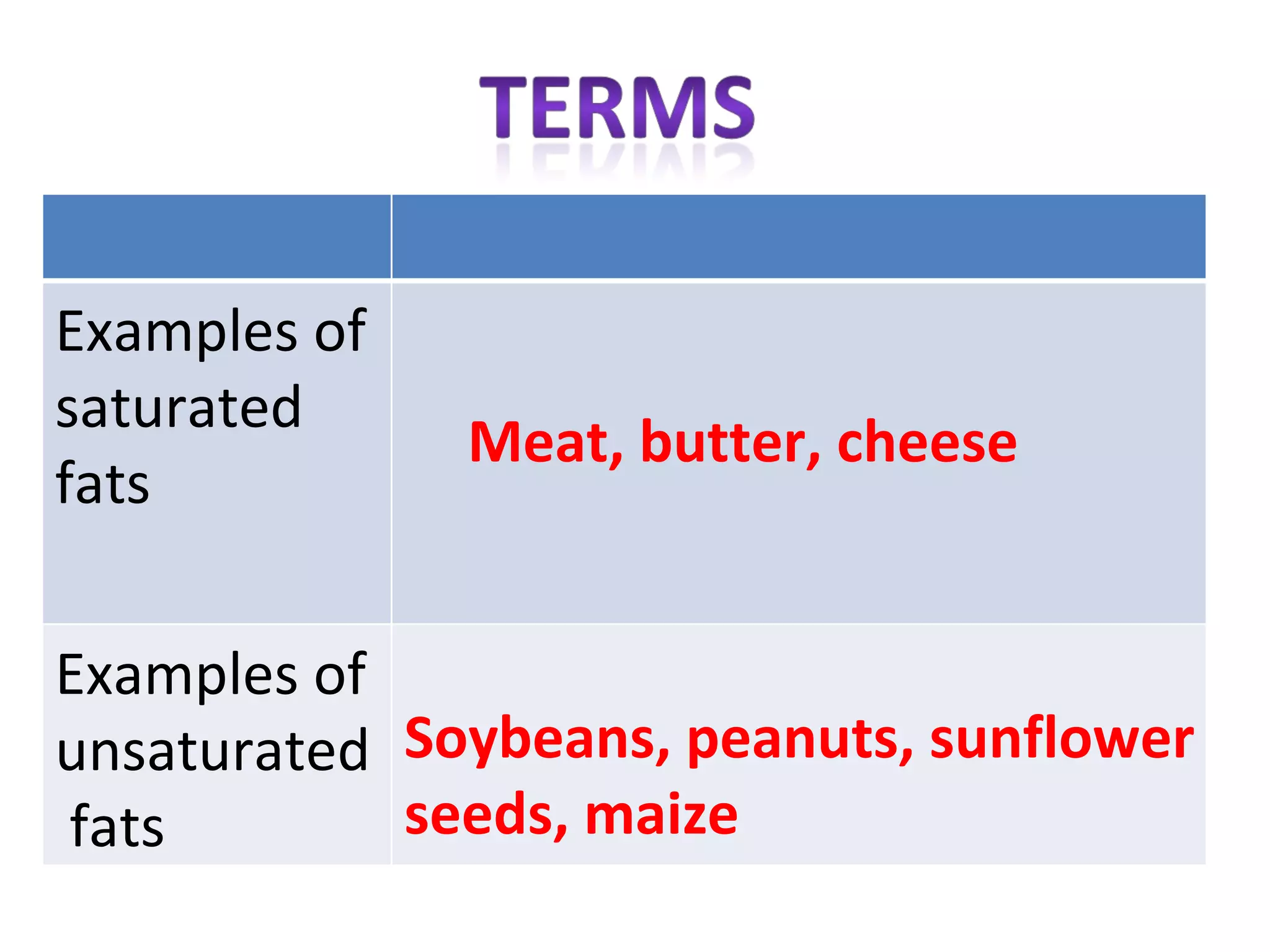
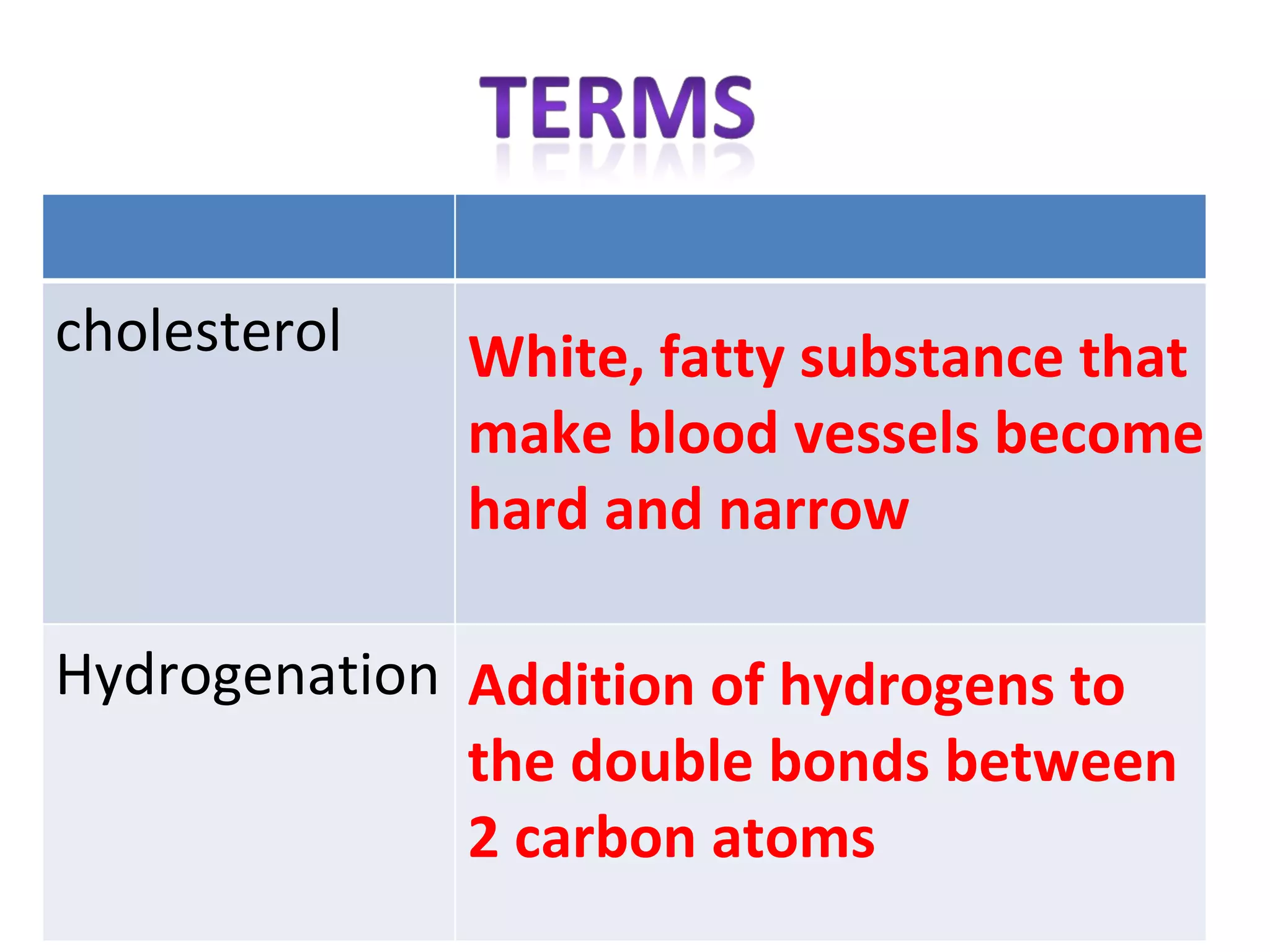
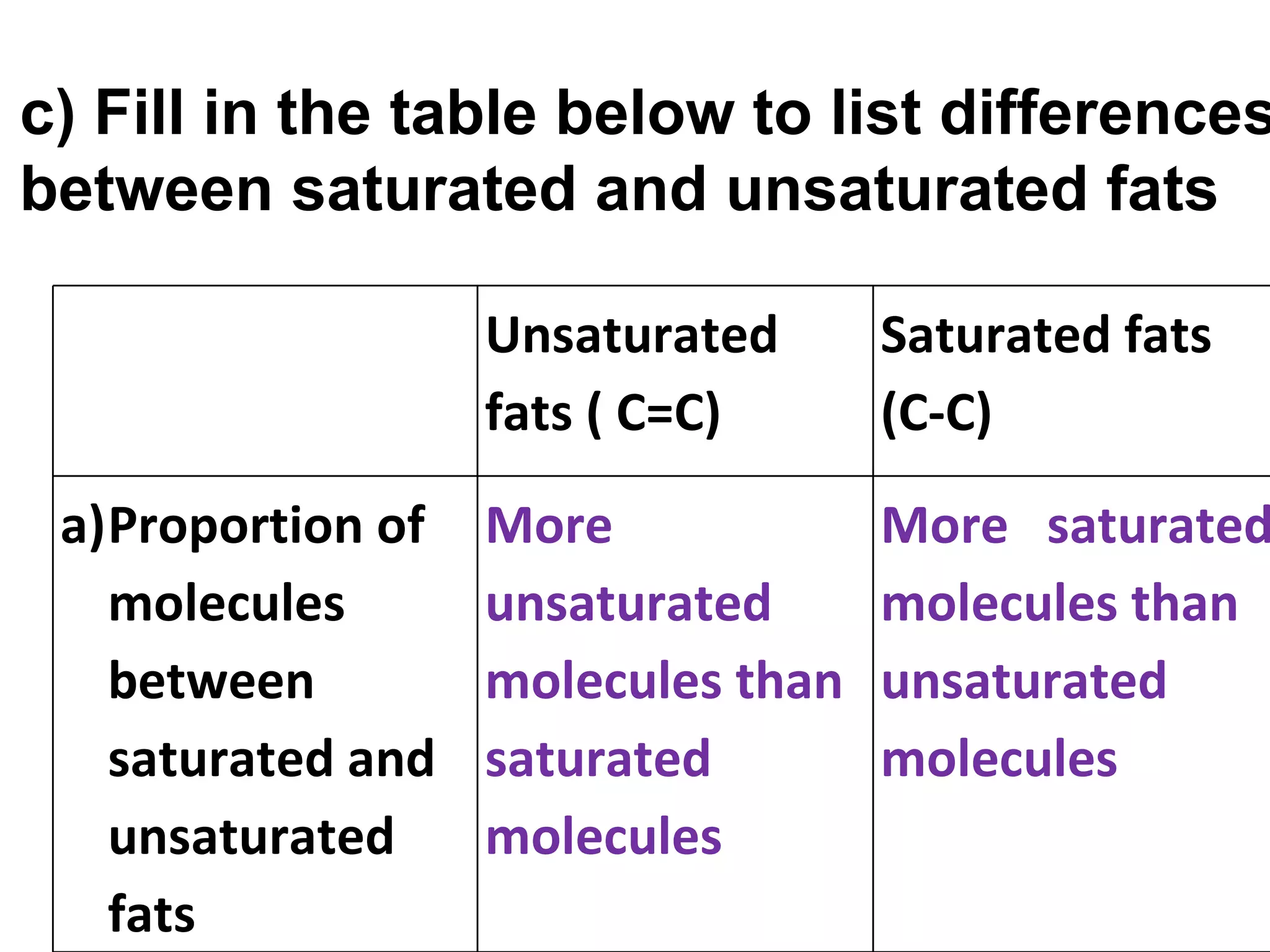
2) Fat molecules contain saturated fatty acids that have no carbon-carbon double bonds, while unsaturated fatty acids contain at least one double bond.
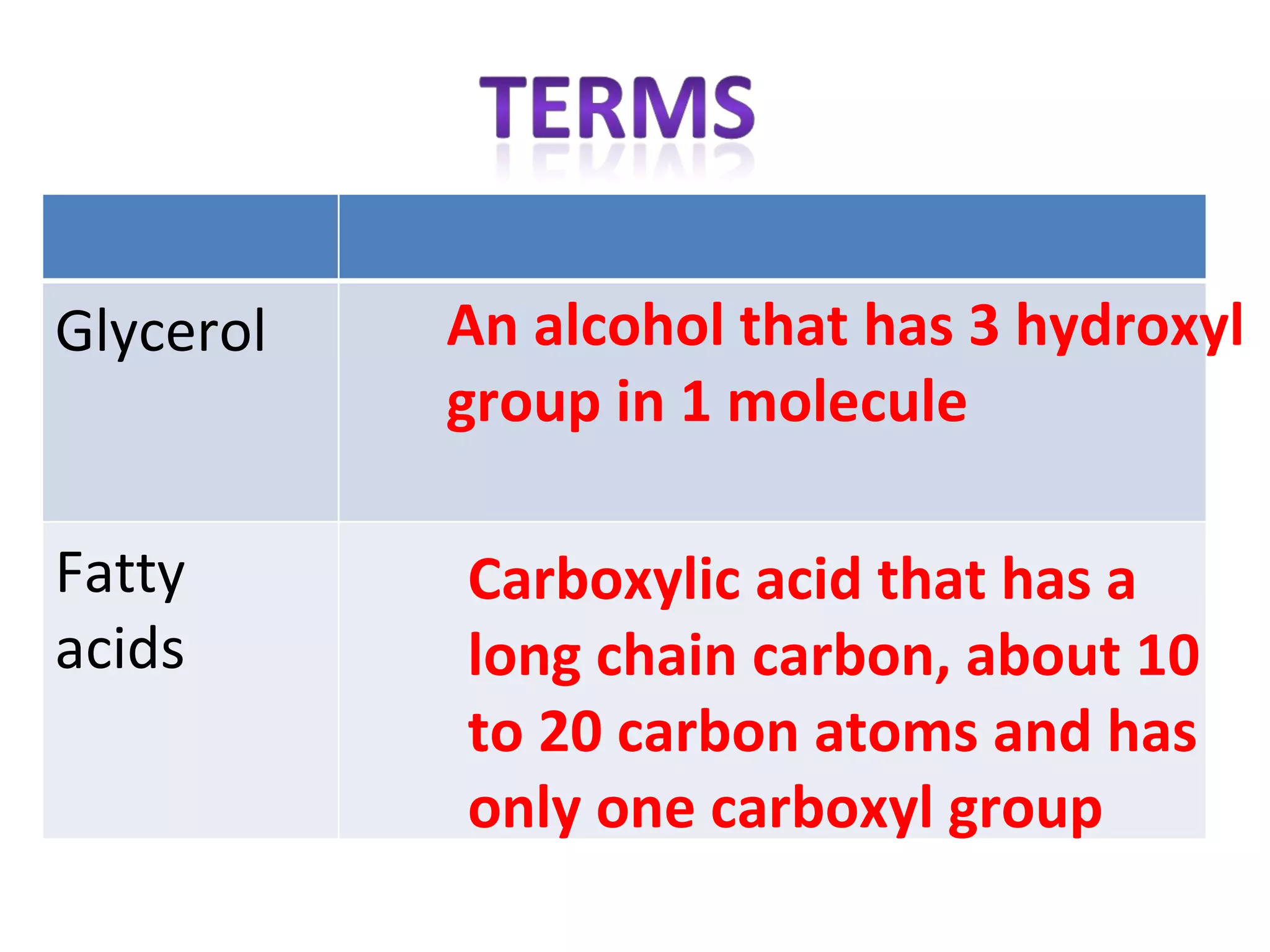
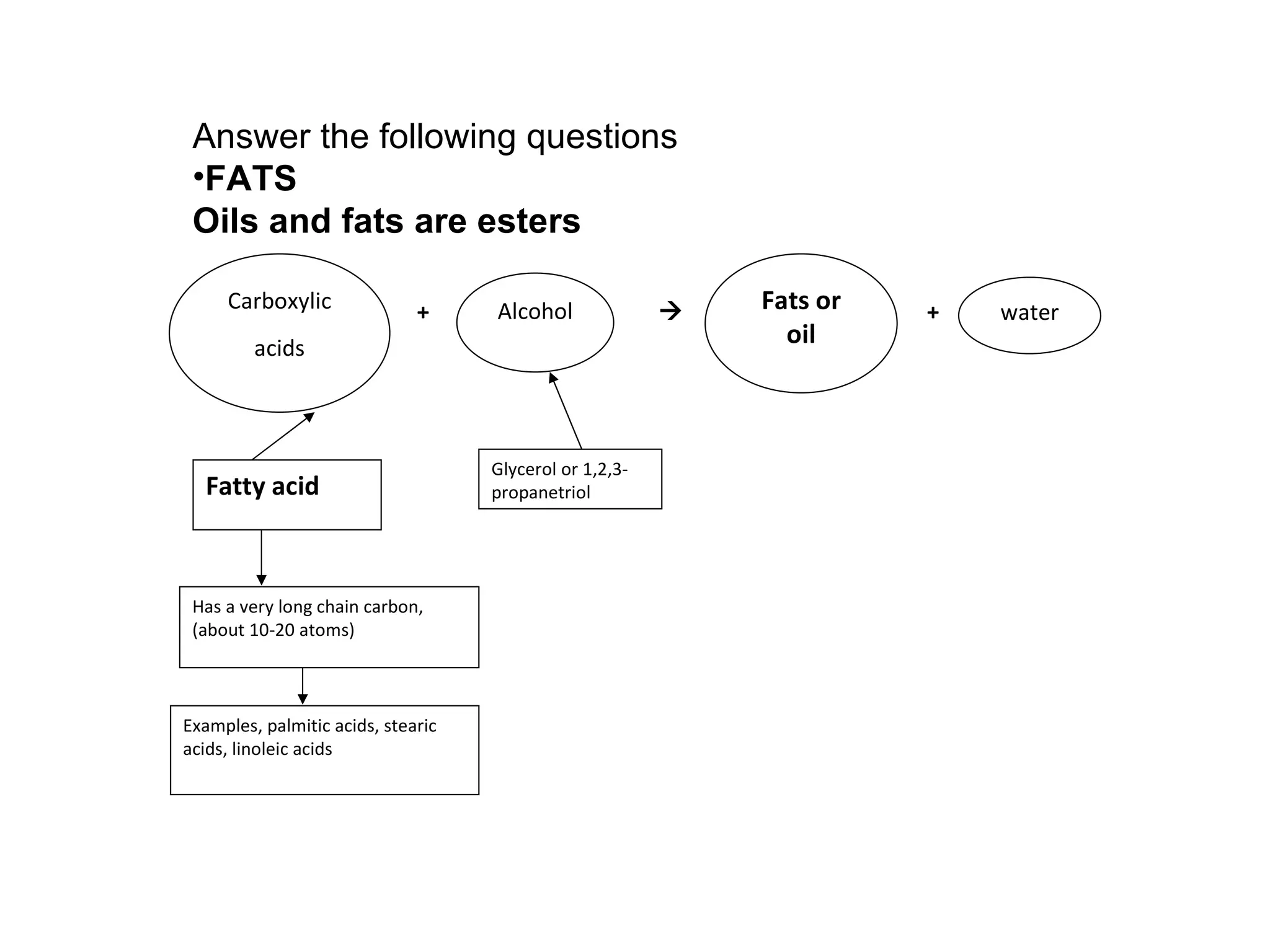
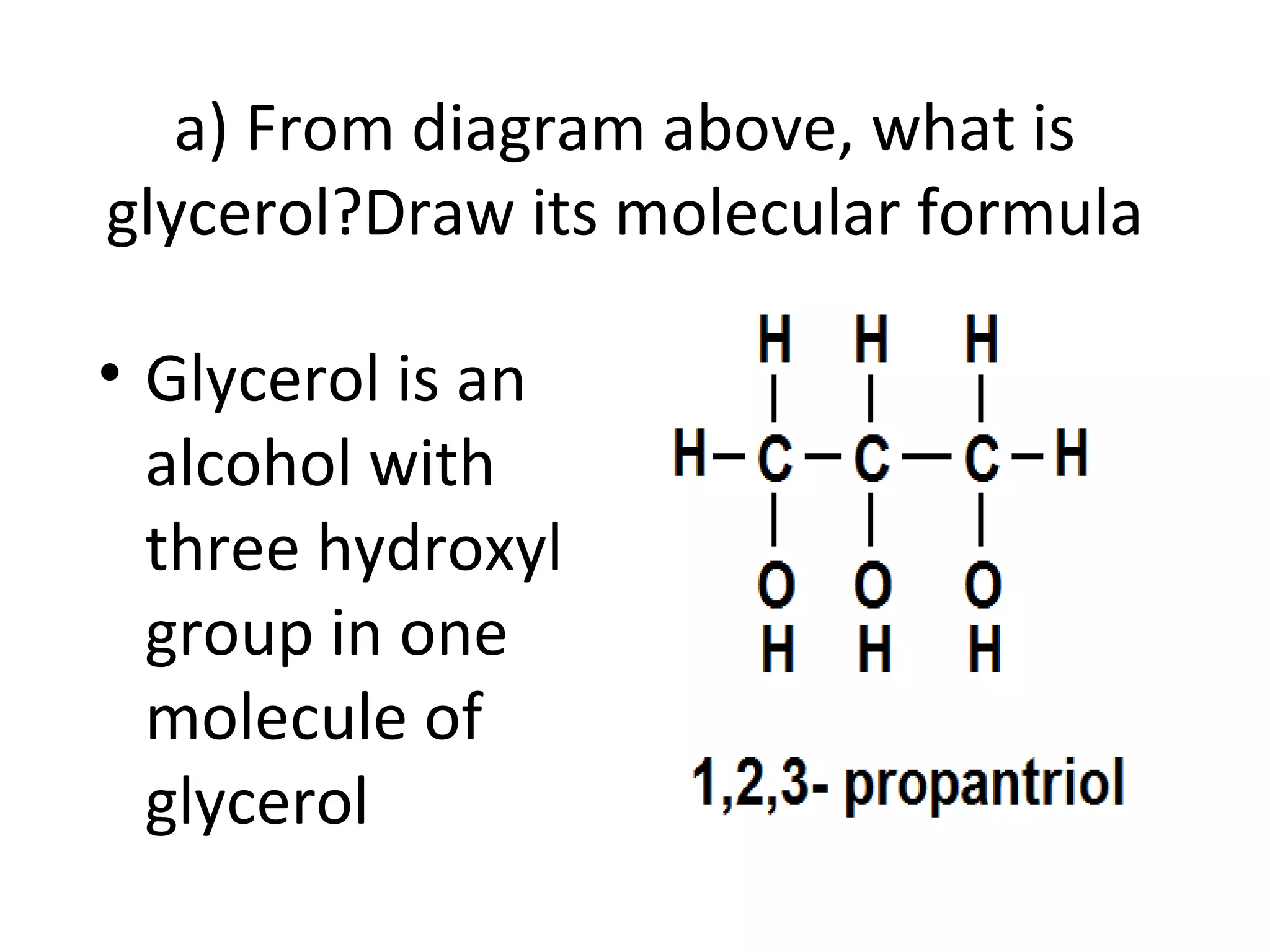
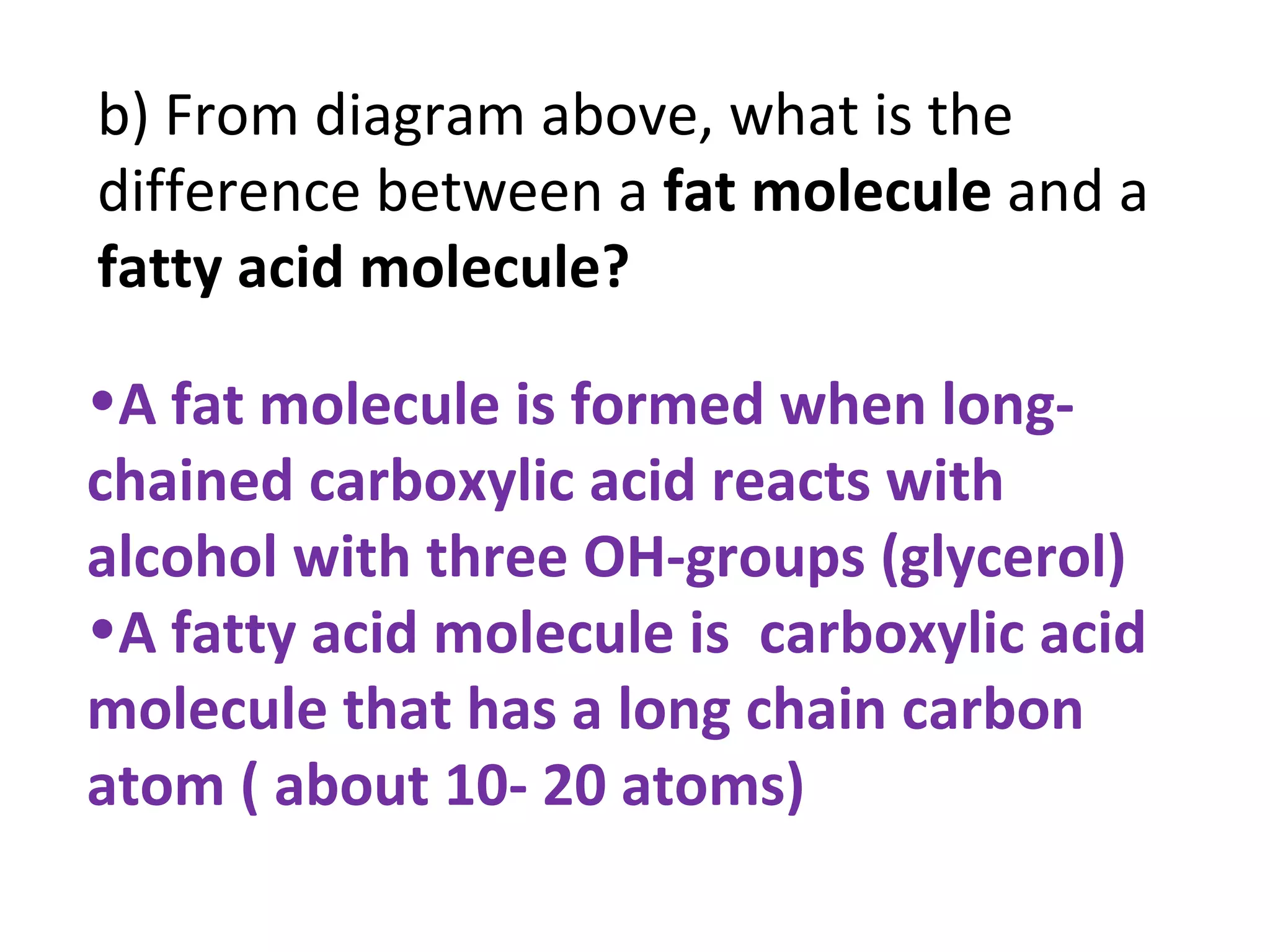
3) Glycerol is an alcohol that reacts with fatty acids to form fat or oil molecules through esterification reactions.