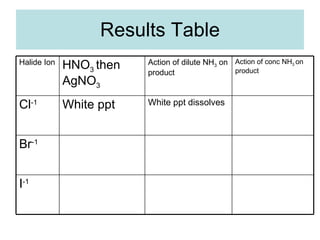
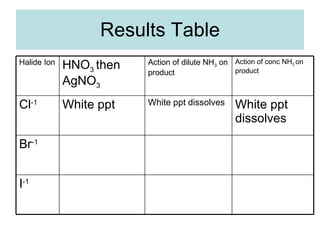
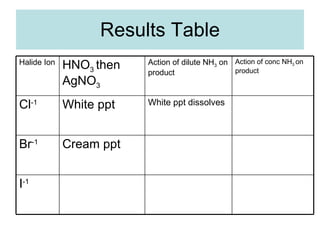
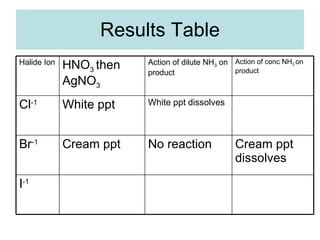
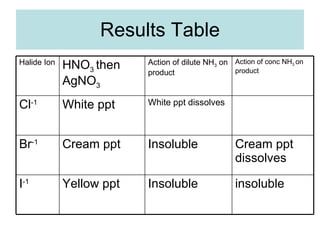
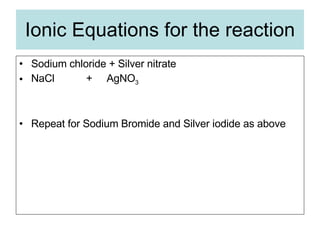
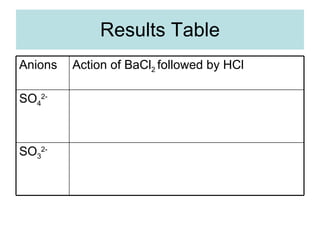
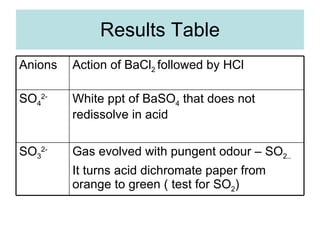
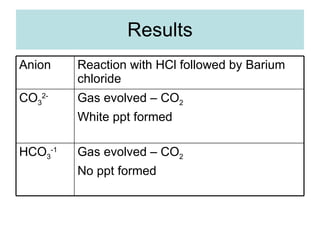
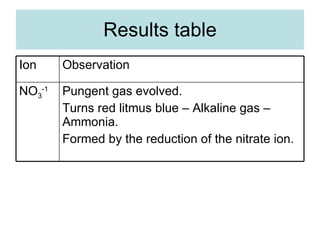
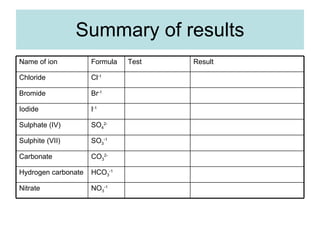
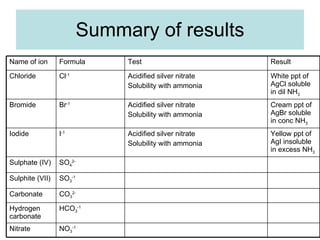
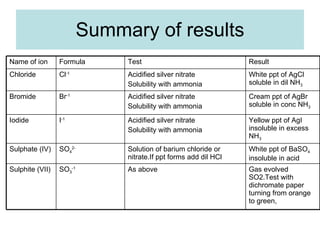
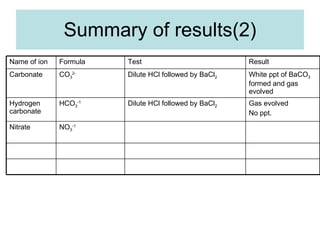
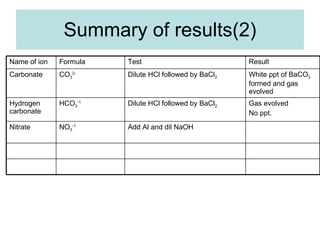
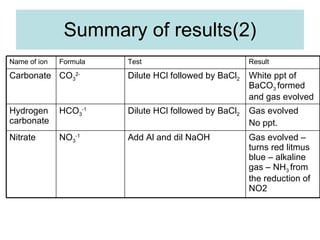
The document describes tests to identify common halide ions and other anions. Chloride, bromide, and iodide ions were tested with silver nitrate and ammonia to observe solubility. Sulphite ions were detected by the production of sulfur dioxide gas detected by dichromate paper. Carbonate and hydrogen carbonate ions were detected through gas production and barium chloride precipitate formation with hydrochloric acid. Nitrate ions were identified by the production of ammonia gas on addition of aluminum and sodium hydroxide.