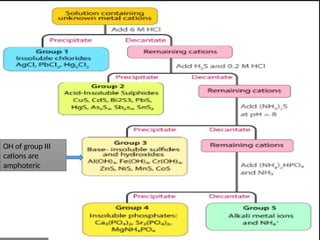
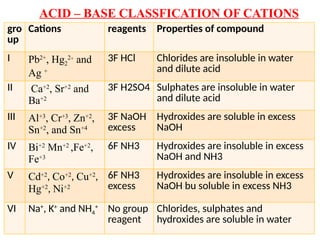
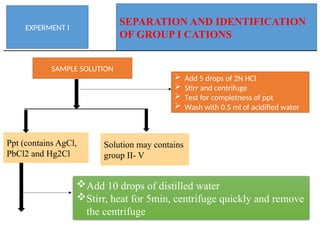
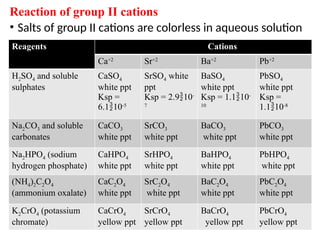
The document discusses qualitative analysis methods for identifying metal ions in salt, detailing various cation groups and their reactions with specific reagents. It outlines the separation of ions through precipitation reactions and identifies techniques for confirming individual cations. Additionally, it describes experimental procedures for grouping and confirming the presence of metal ions, focusing on the different solubility characteristics of their compounds.





![Solution (may contains Pb2+
)
Add K2Cro4, yellow ppt
PbCrO4 the presence of Pb2+
Ppt (may contain AgCl and Hg2Cl2
Solution( may ontain [Ag(NH3)2]+
)
Add drops of 6N HNO3
Repreciptation of AgCl confirms Ag+
Add 10 drops of 2N NH3
Stirr and centrifuge
Precipitate (contains Hg(NH2)Cl + Hg
Black or gray ppt of Hg(NH2)Cl + Hg
Confirms the presence of Hg](https://image.slidesharecdn.com/ppchem223practical-241101060034-8f7135a0/85/practical-analytical-chemistry-ChEd-2352-power-point-presentation-10-320.jpg)
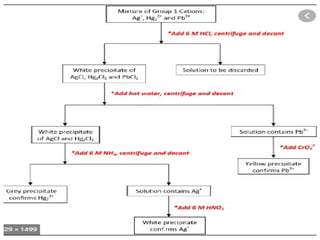
![REACTION OF GROUP I CATIONS
colorless aqueous solution of group I catain forms chloride salt
which are slightly soluble in water, dilute nitric and sulphuric acid.
Whe dilute HCl is added to solution of salt containing Pb2+
, Hg2
2+
and Ag +
cations white ppts of PbCl2 , Hg2Cl2 and AgCl is formed.
Solubility of PbCl2 , Hg2Cl2 and AgCl can be decreased by a slight
excess HCl or Cl-
(common ion effect).
Large amount of Cl-
causes dissociation of PbCl2 , Hg2Cl2 and AgCl
due to formation of soluble [AgCl3]2-
, [PbCl4]2-
and 2[HgCl2
]-
Complexes
Ag +
+ Cl-
AgCl Ksp=1.5610-10
Almost Completely ppt
Hg2
2+
+ Cl-
Hg2Cl2 Ksp=210-18
Almost Completely ppt
Pb2+
+ Cl-
PbCl2 Ksp=110-4
Relatively soluble ( no more ppt is
formed)](https://image.slidesharecdn.com/ppchem223practical-241101060034-8f7135a0/85/practical-analytical-chemistry-ChEd-2352-power-point-presentation-11-320.jpg)
![ Separation of PbCl2 from Hg2Cl2 and AgCl is based on solubility in
water. PbCl2 Is completely dissolved in hot water.
Separation of AgCl from Hg2Cl2 and PbCl2is based on solubility in
aqueous NH3.
AgCl + 2NH3 [Ag(NH3)2]+
+ Cl-
The complex is stable in excess NH3. if the solution containing
[Ag(NH3)2]+
and Cl-
is acidified with nitric acid or with any acid
which does not form ppt with Ag+
ion the complex is destroyed and
white AgCl is re-precipitated.
[Ag(NH3)2]+
+ Cl-
+ 2H3O+
AgCl + 2NH4
+
+2H2O
Hg2Cl2Is not soluble in queous NH3 but forms unstable and rapidly
decomposable [NH2Hg2]Cl (amido complex).
Hg2Cl2 + 2NH3 [NH2Hg2]Cl + Cl-
+ NH4
+
[NH2Hg2]Cl [NH2Hg]Cl + Hg (black ppt)
Blackening of ppt occurs due to the formation of metallic mercury.](https://image.slidesharecdn.com/ppchem223practical-241101060034-8f7135a0/85/practical-analytical-chemistry-ChEd-2352-power-point-presentation-12-320.jpg)
![Some reaction of group I cations
reagent Cations
Ag+
Pb2+
Hg2
2+
3F HCl or soluble
chlorides (slight
excess)
White pp AgCl White ppt PbCl2 White ppt Hg2Cl2
3F H2SO4 or
soluble sulphates
No ppt is formed White ppt PbSO4 White ppt Hg2SO4
Aqueous NH3
(excess)
Colorless solution
[Ag(NH3)2]+
White ppt
Pb(OH)2
Black or gray ppt
[NH2Hg]X (white) +
Hg (black)
NaOH (no
excess)
brown ppt Ag2O White ppt
Pb(OH)2
brown ppt Hg2O
NaOH (excess) brown ppt Ag2O Colorless solution
[Pb(OH)4]2-
brown ppt Hg2O](https://image.slidesharecdn.com/ppchem223practical-241101060034-8f7135a0/85/practical-analytical-chemistry-ChEd-2352-power-point-presentation-13-320.jpg)
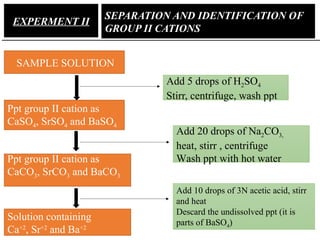
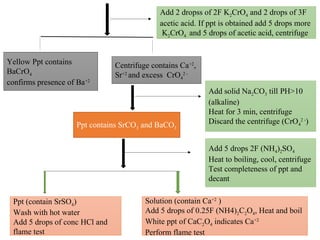
![• Pb is precipitated as chloride in group I and as sulphate in
group II
• Solubility of lead sulphate in hot alkaline solution is used
to separate lead ion fromg roup II cation.
PbSO4 + 4OH-
[Pb(OH)4]2-
+ SO4
2-
• Flame tests of some group II cations
Brick red (Ca) Crimson red Yellow green](https://image.slidesharecdn.com/ppchem223practical-241101060034-8f7135a0/85/practical-analytical-chemistry-ChEd-2352-power-point-presentation-17-320.jpg)
![SEPARATION AND IDENTIFICATION OF
GROUP III CATIONS
Experment
III
Sample solution
Add 2N NaOH excess,
add 3%H2O2 while
stirring Heat for 5min,
centrifuge
Solution containing
[Zn(OH)4]2-
,[Al(OH)4]-
,
[Sn(OH)6]2-
and CrO4
2-
The ppt contains group IV and V
Add solid NH4Cl and stirr till you get the
characterstics of smells of ammonia heat
and centrifug](https://image.slidesharecdn.com/ppchem223practical-241101060034-8f7135a0/85/practical-analytical-chemistry-ChEd-2352-power-point-presentation-18-320.jpg)