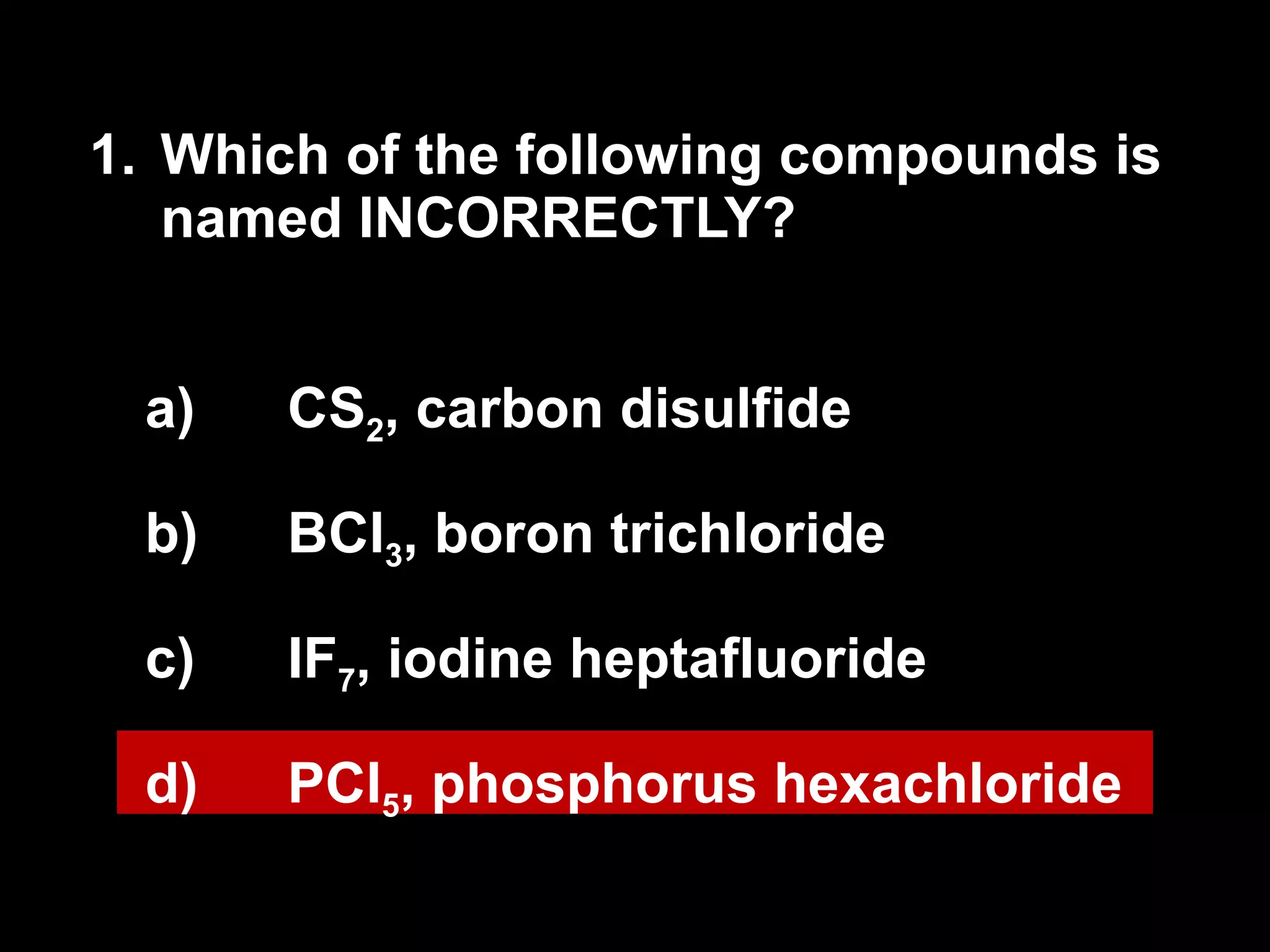
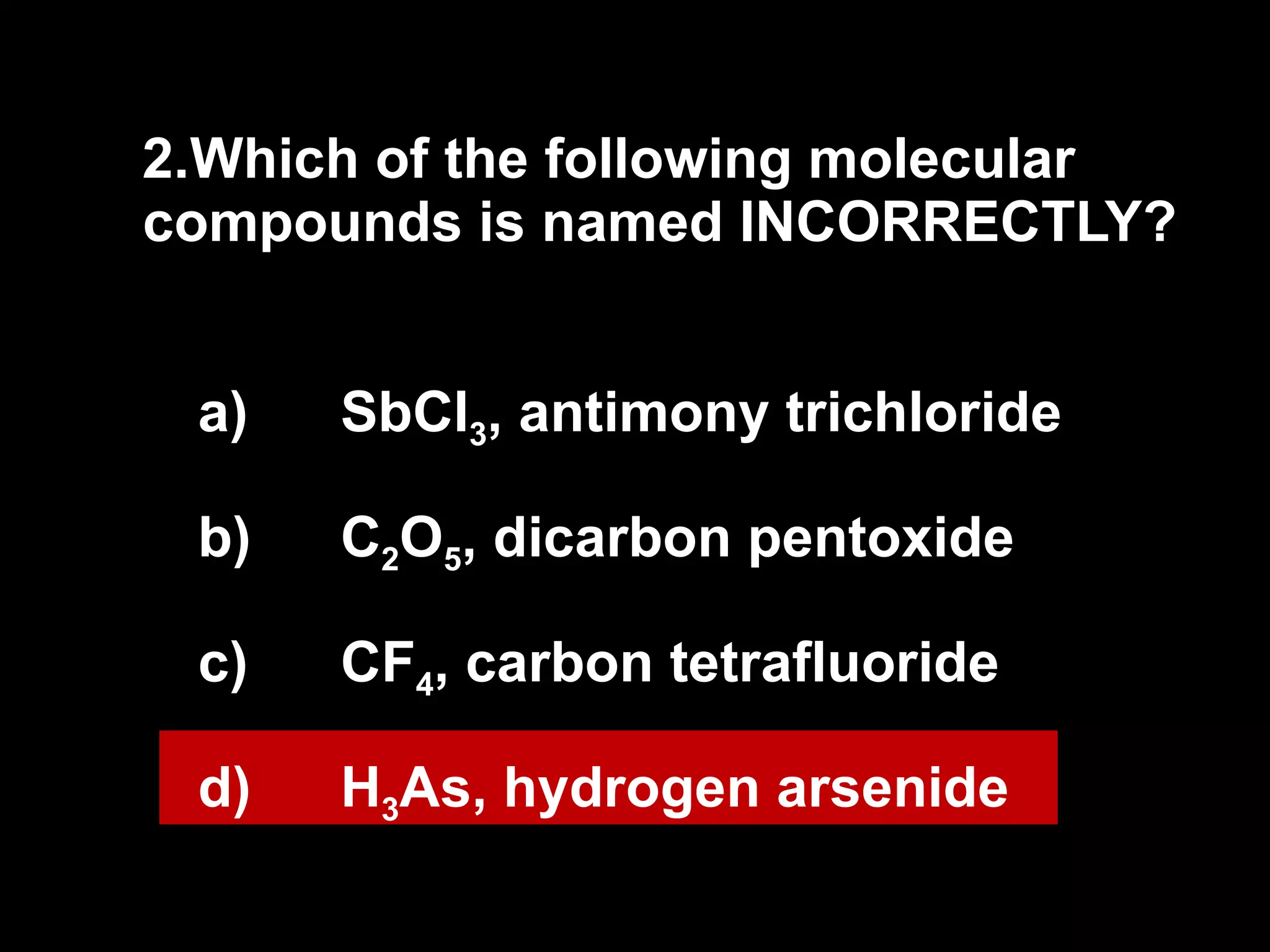
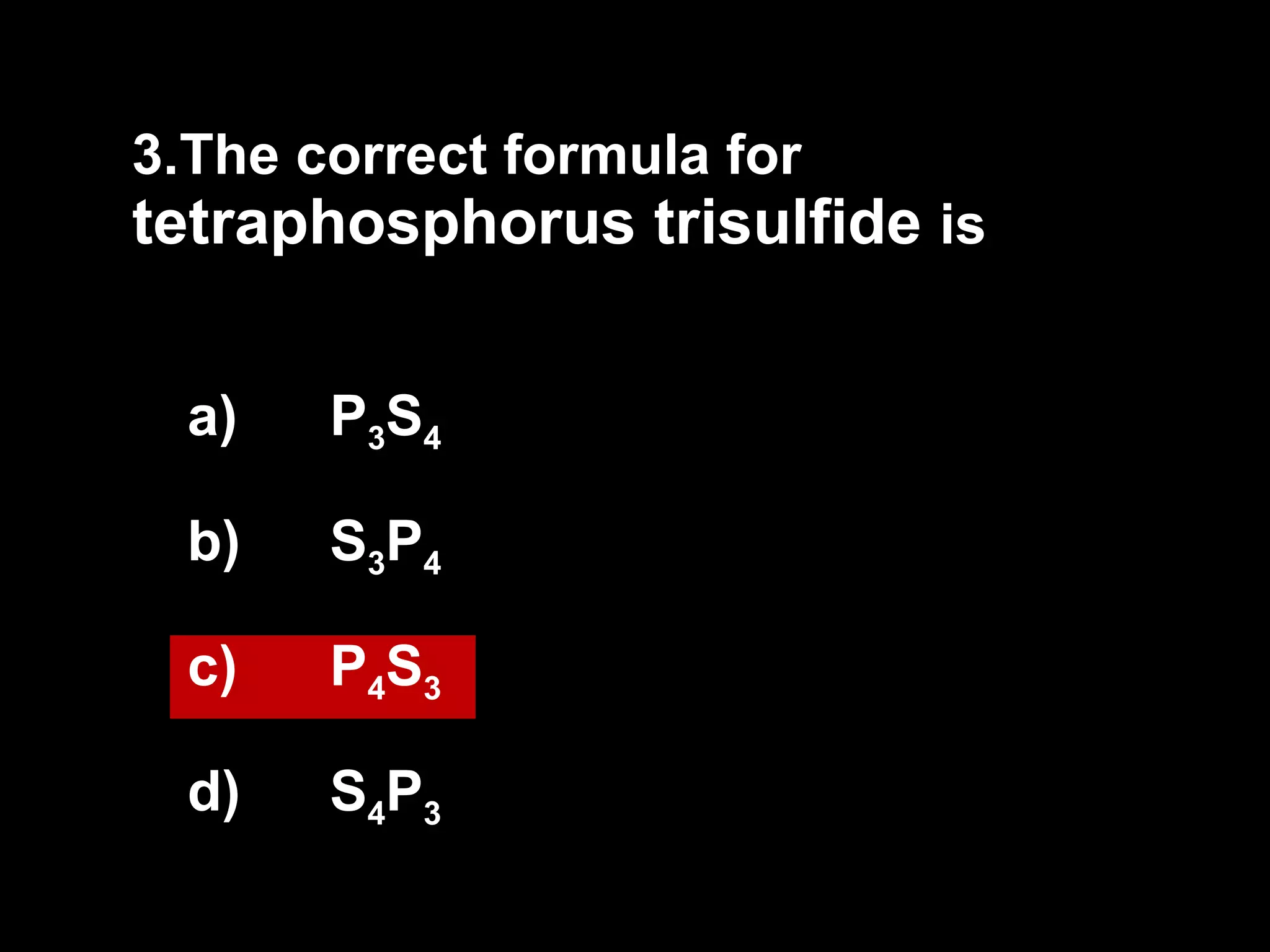
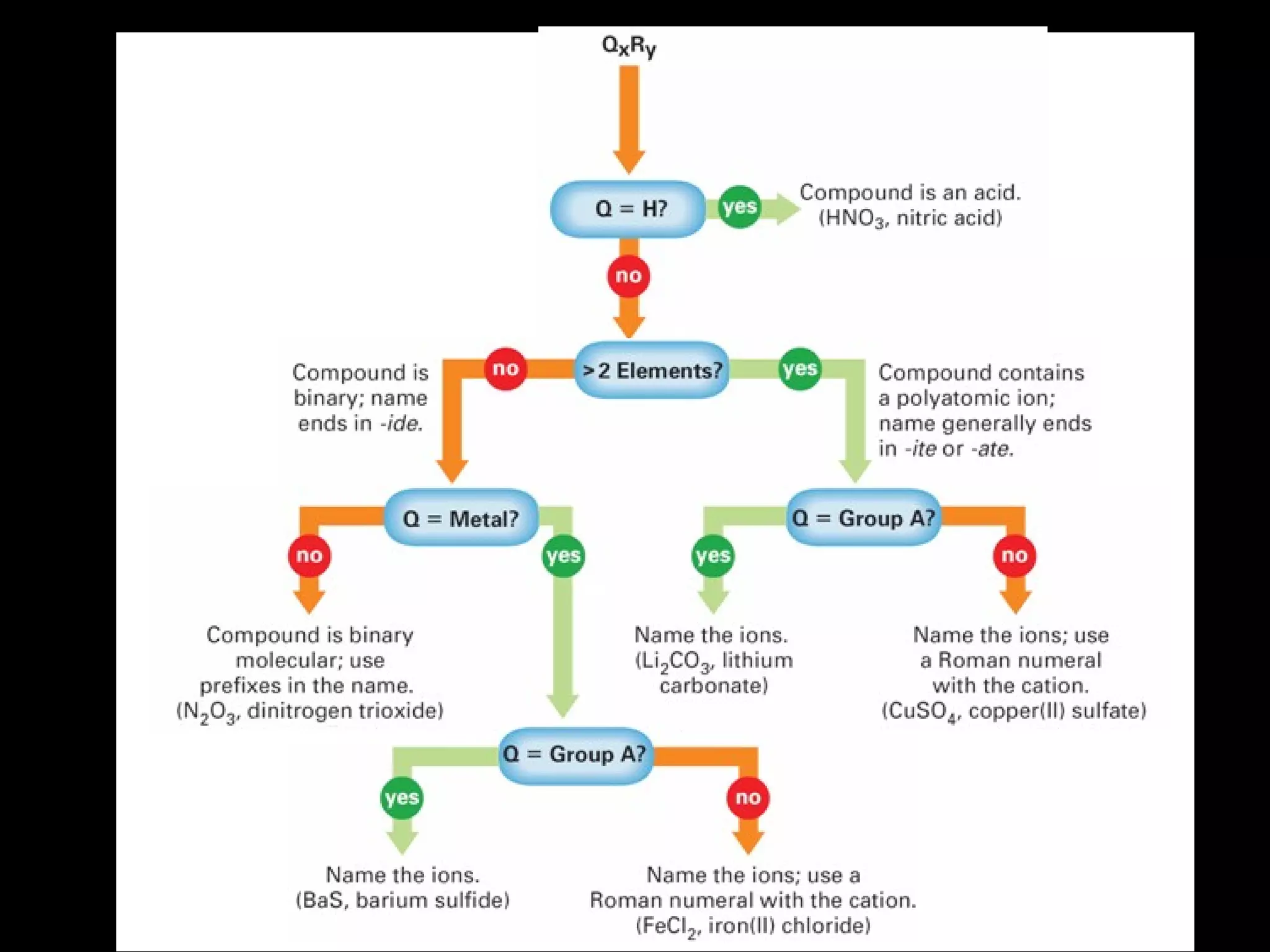
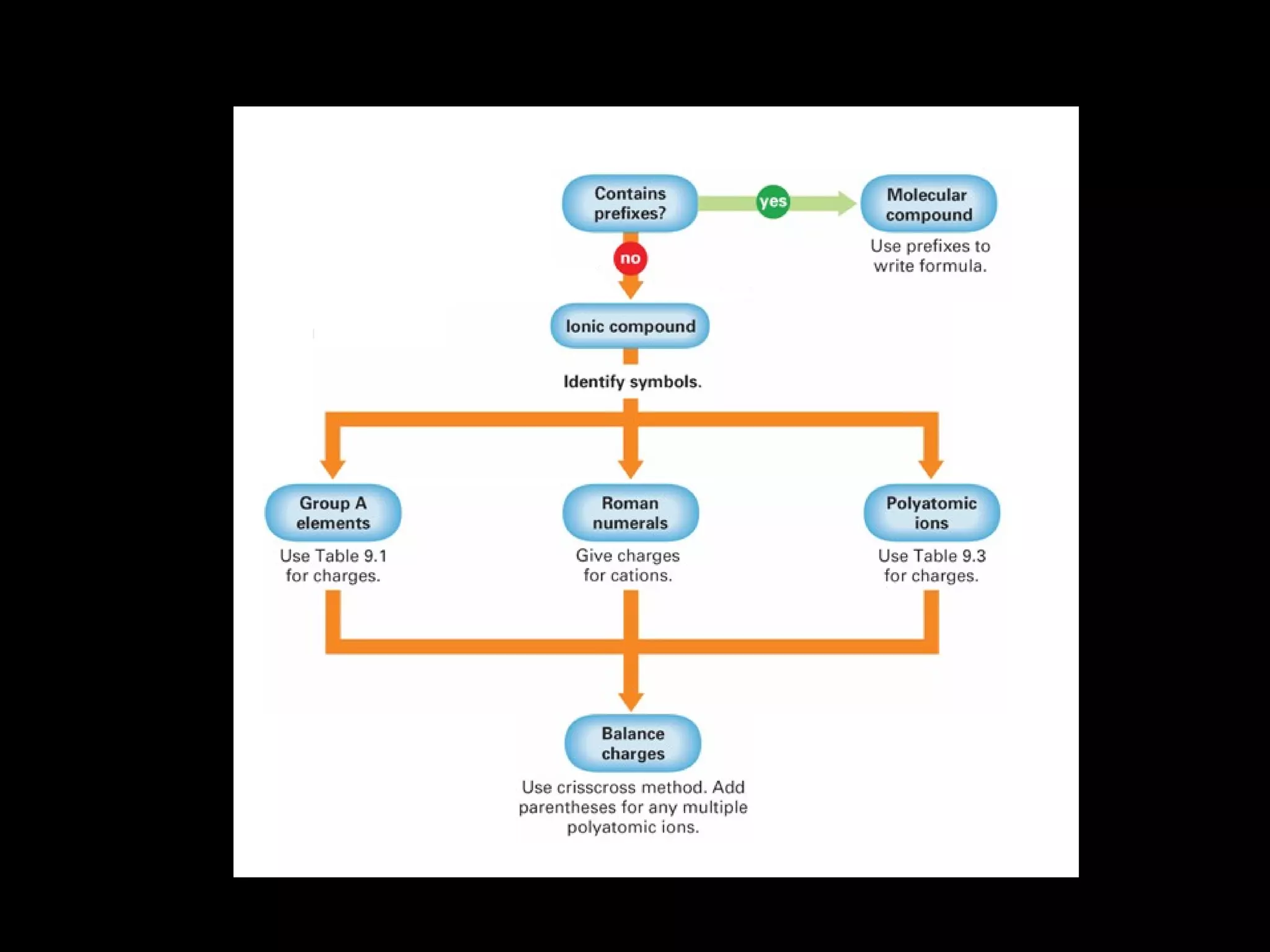
The document discusses naming binary molecular compounds and acids and bases. It provides guidelines for naming compounds based on their formulas or vice versa. The key points are:
- Binary molecular compounds are named with prefixes to indicate the number of atoms and the suffix "-ide" for the second element.
- Acids are named after their anions and have formulas starting with hydrogen and ending with the anion formula. Their names indicate the anion stem.
- Bases are named by the cation name followed by the hydroxide anion name and have the hydroxide formula.
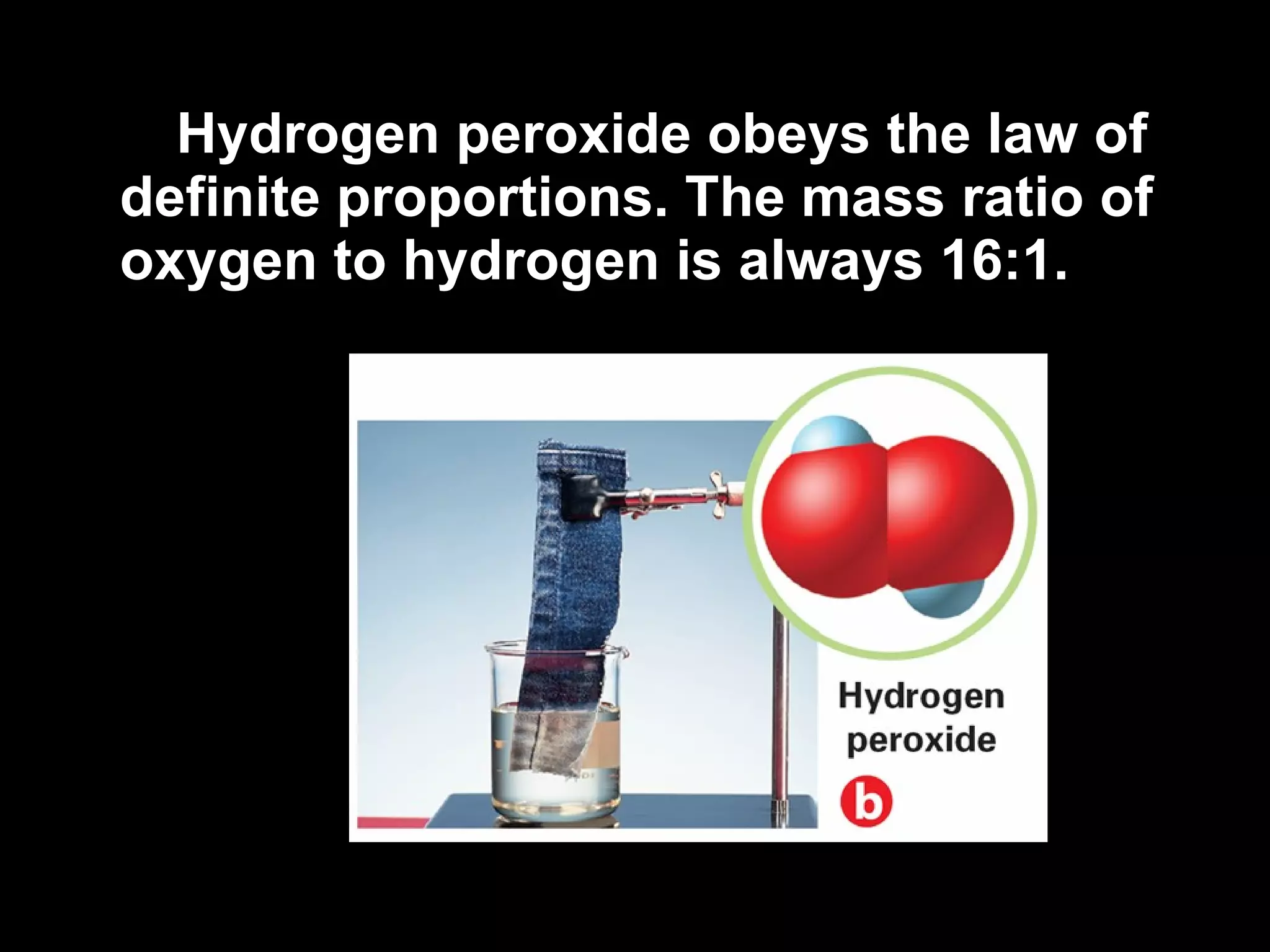
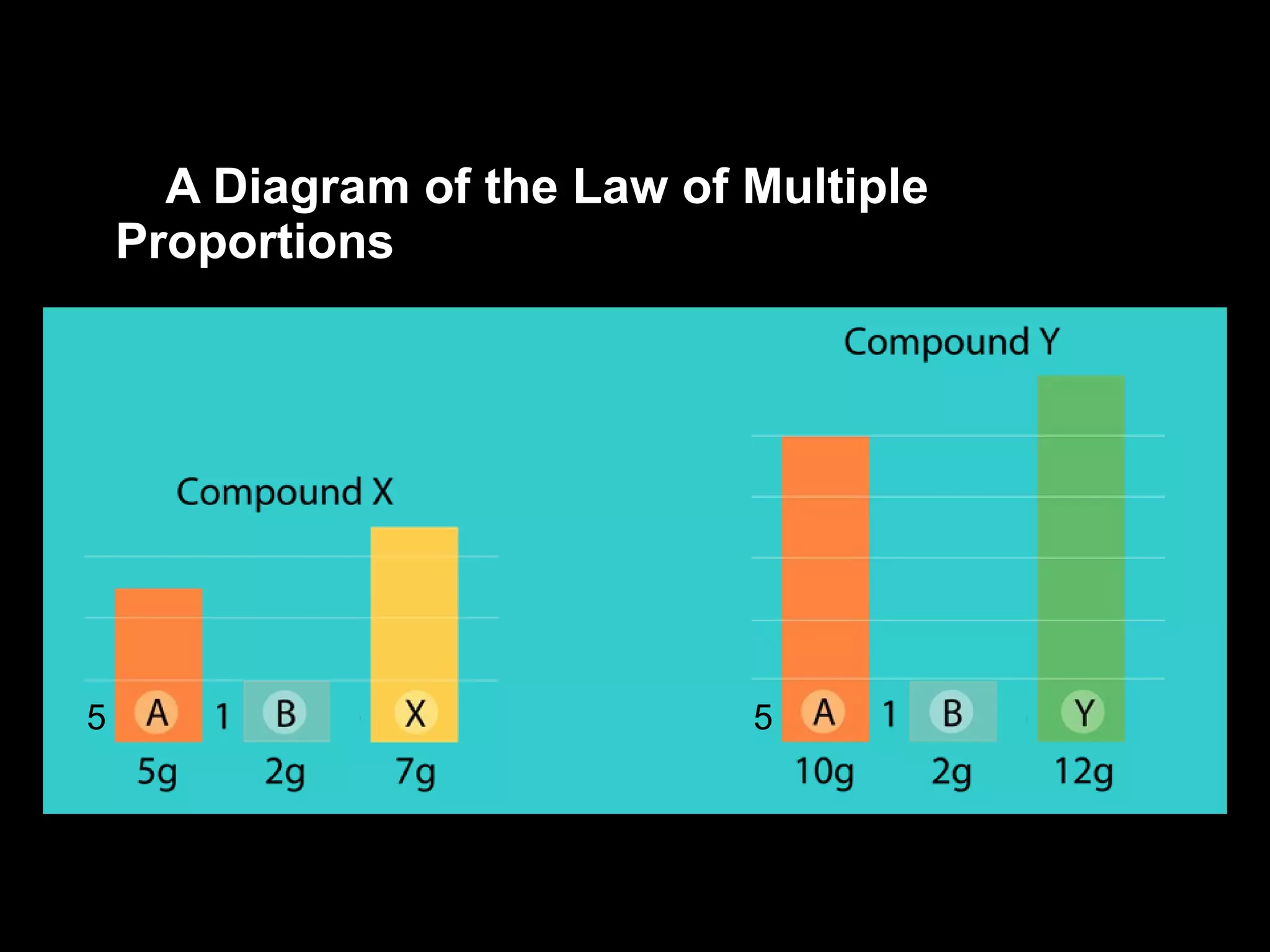
- The laws of definite and multiple proportions describe the consistent element ratios in compounds.