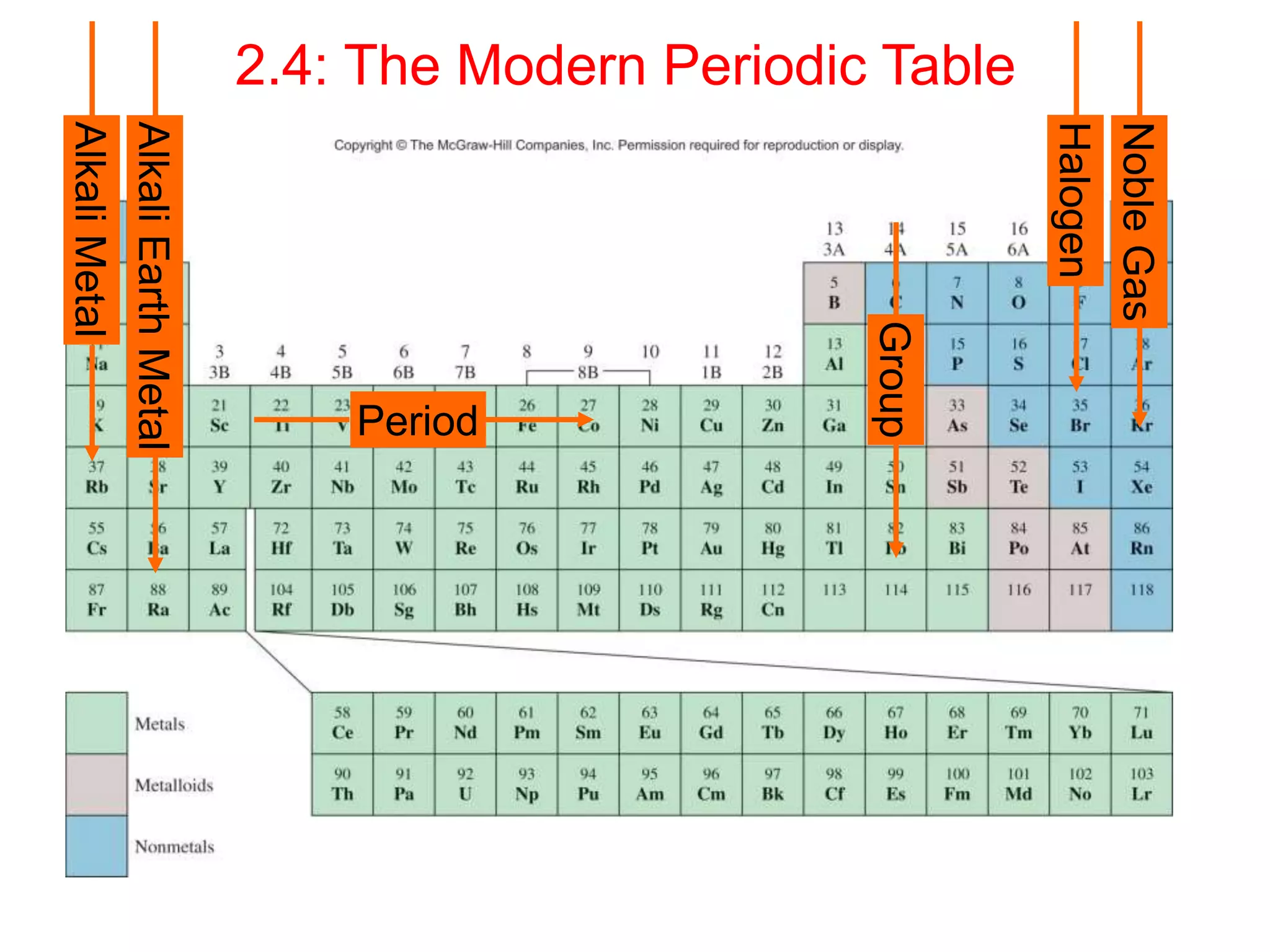
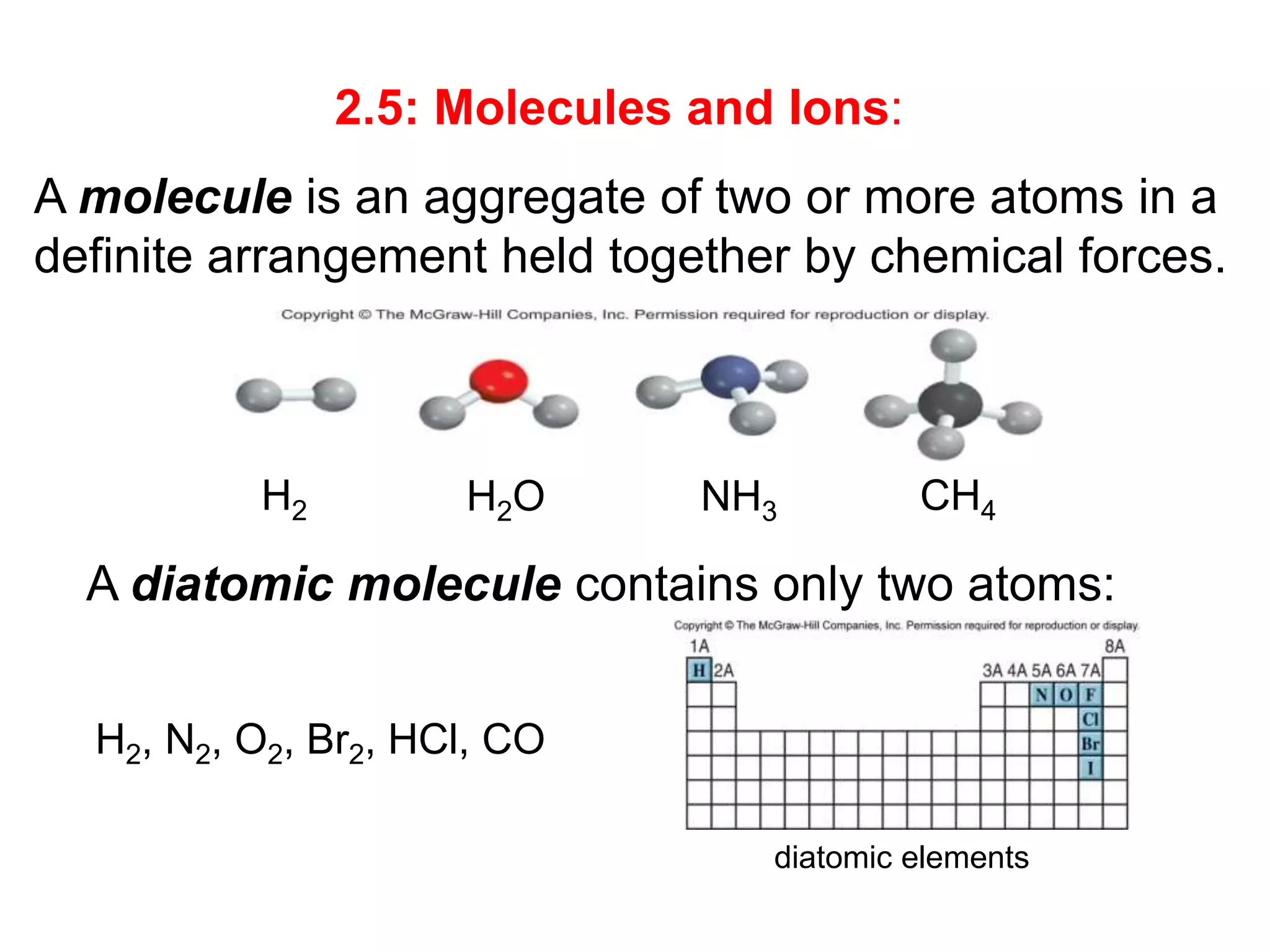
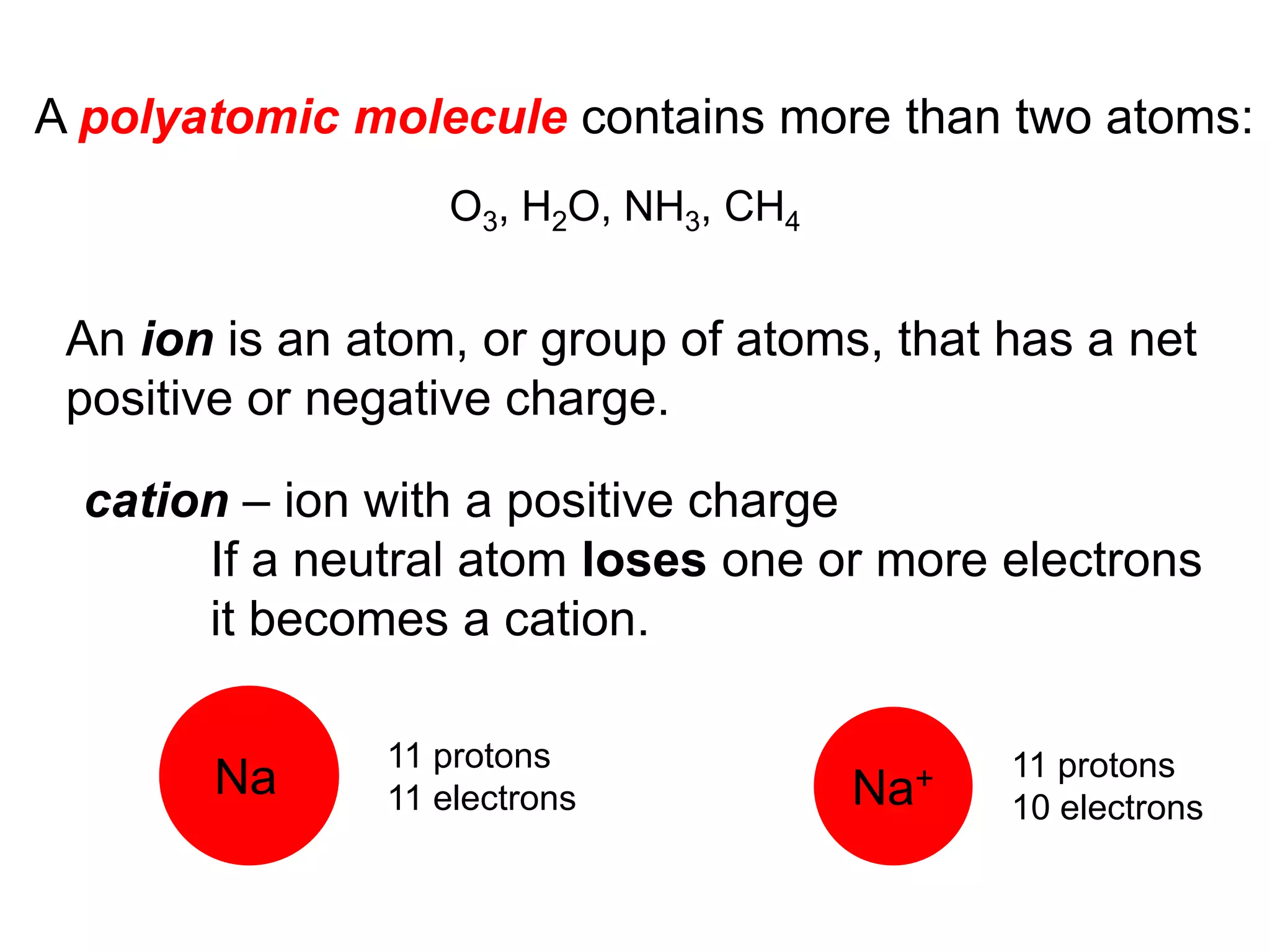
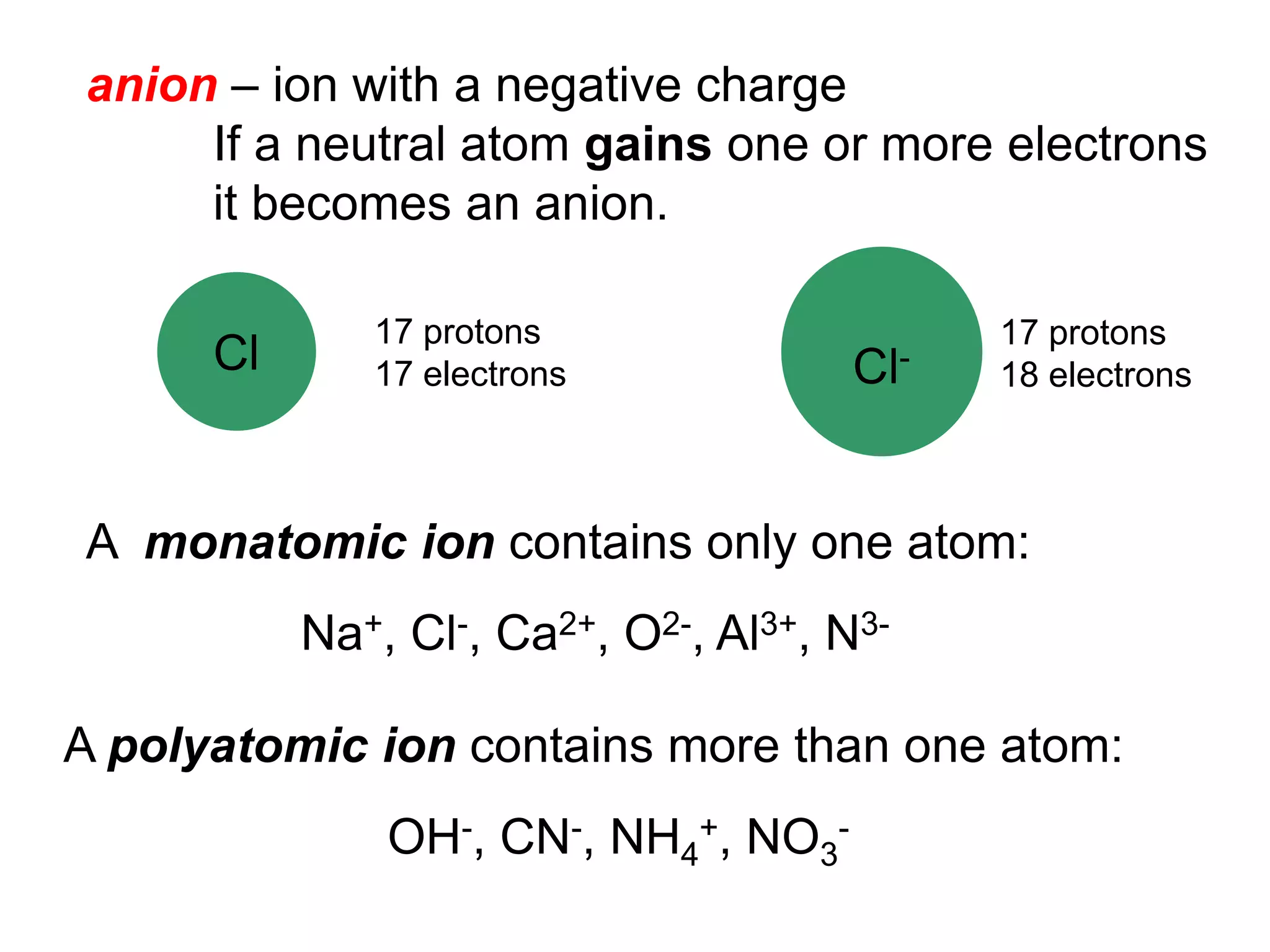
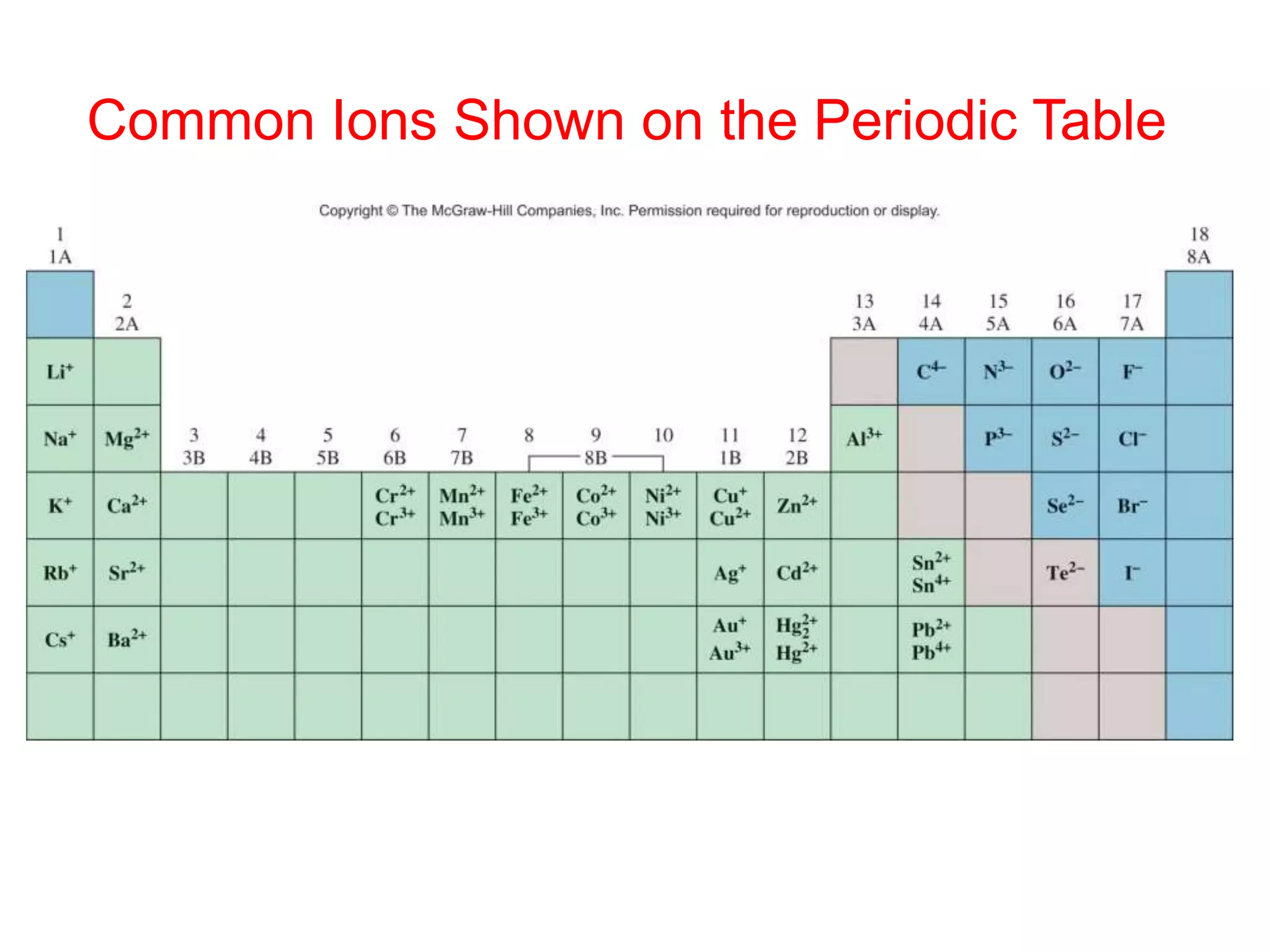
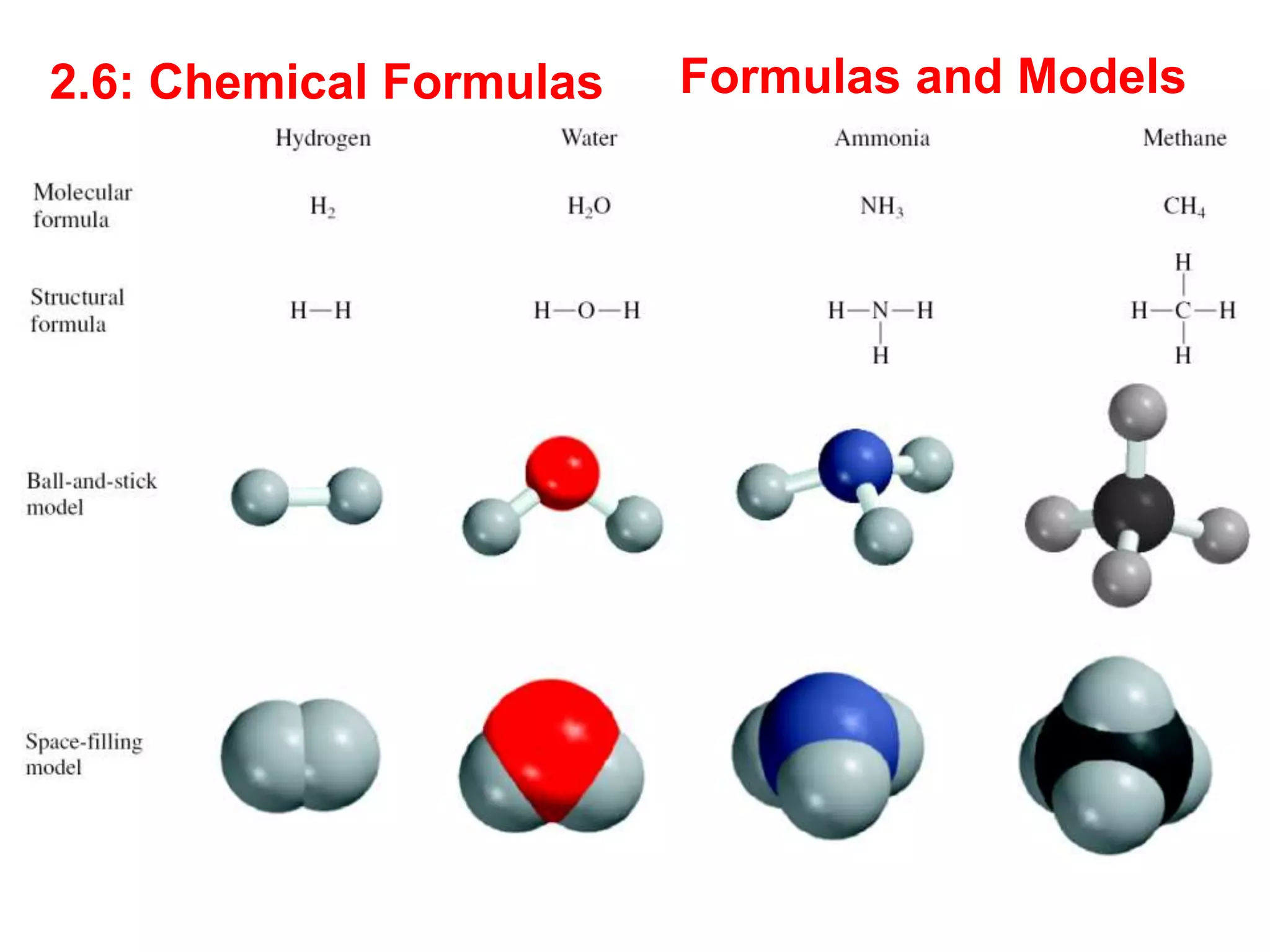
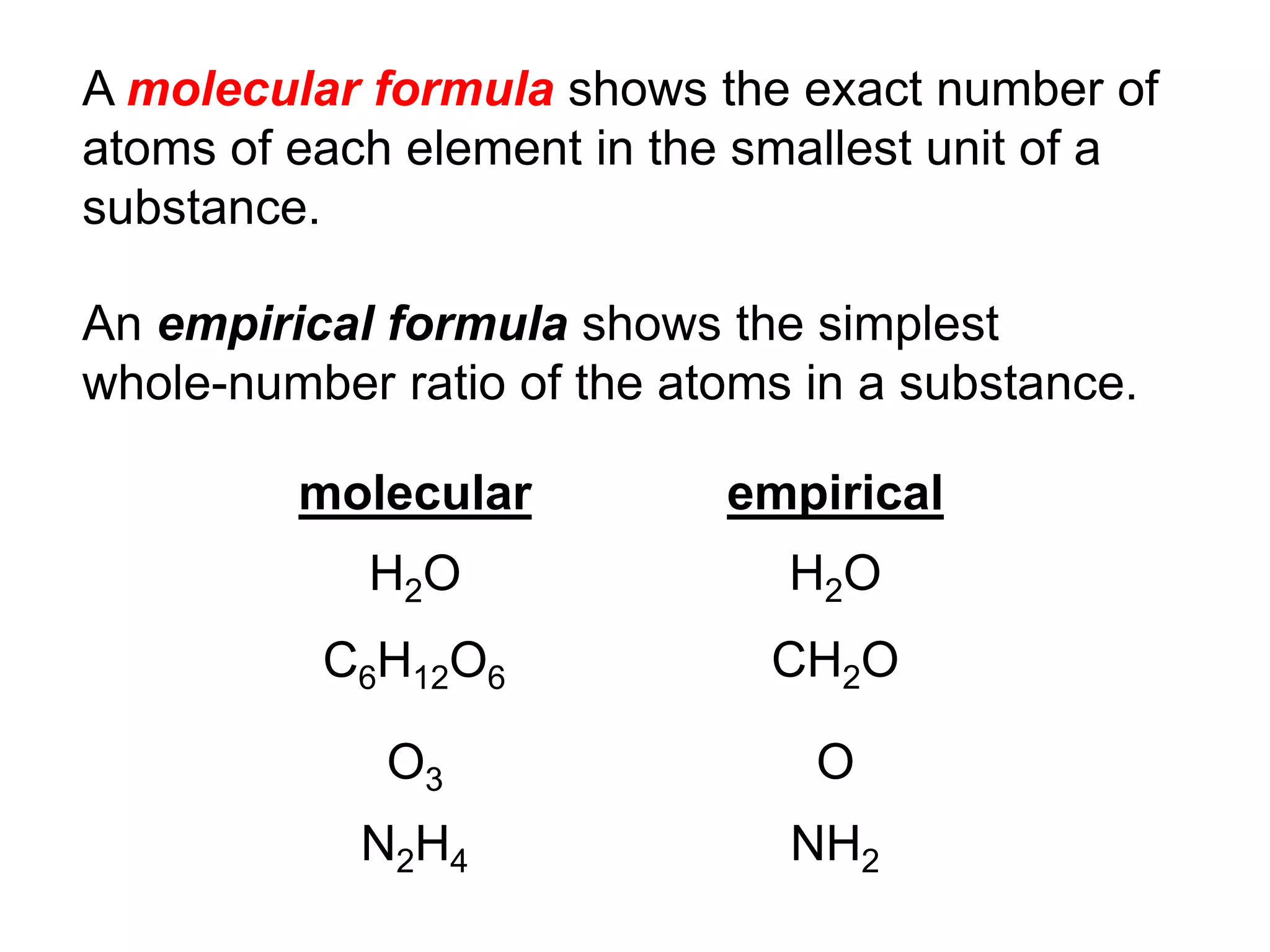
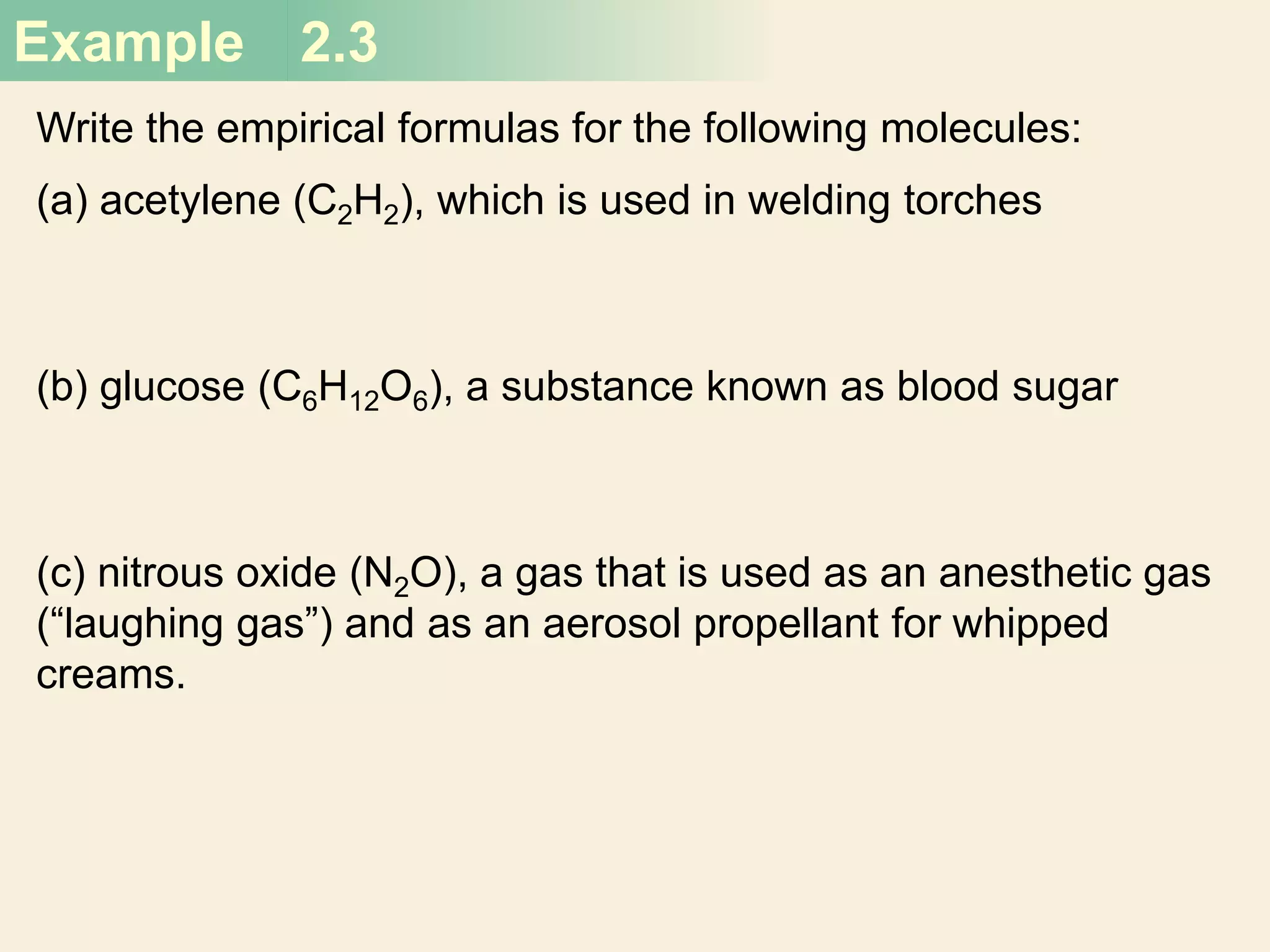
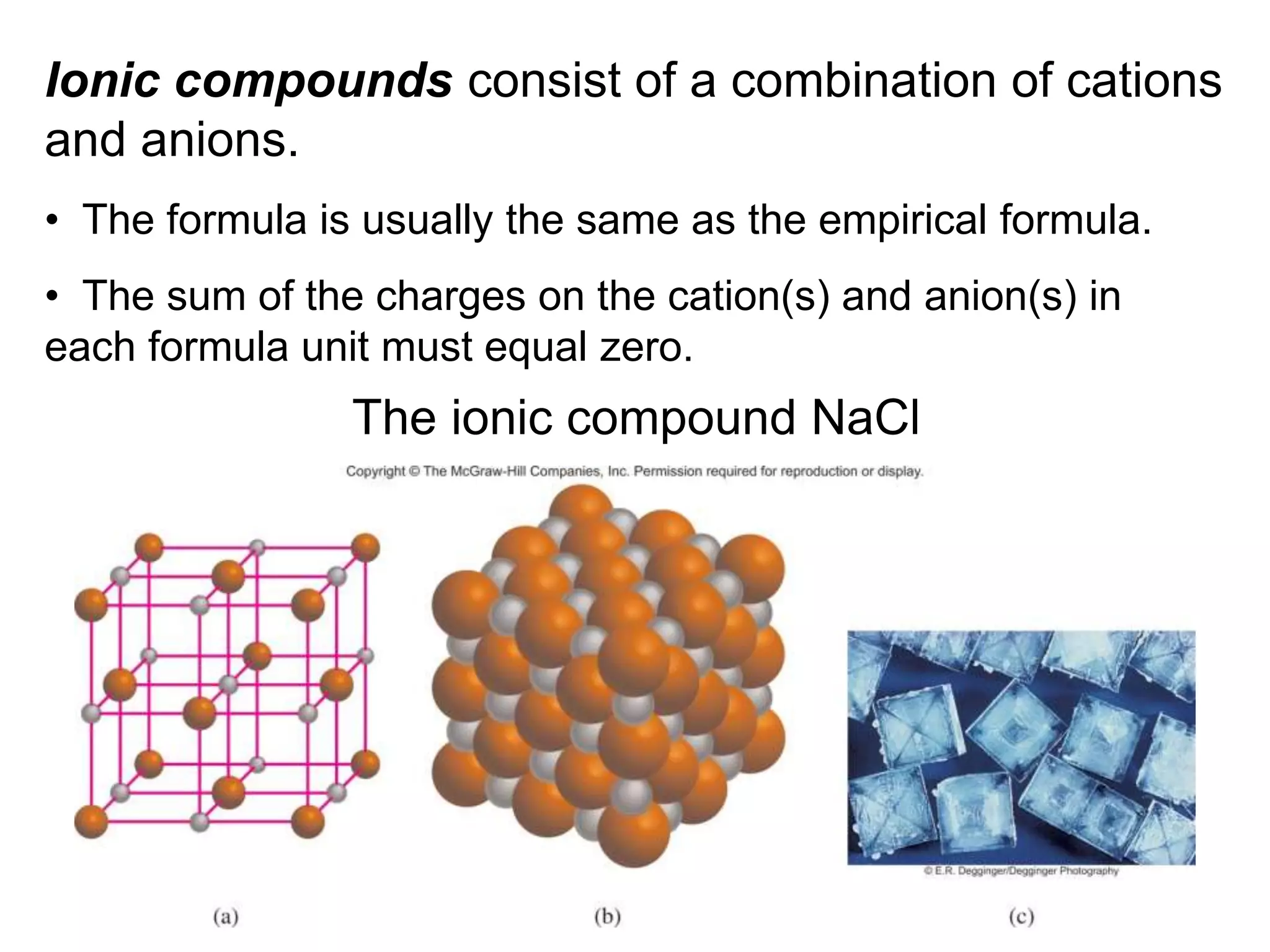
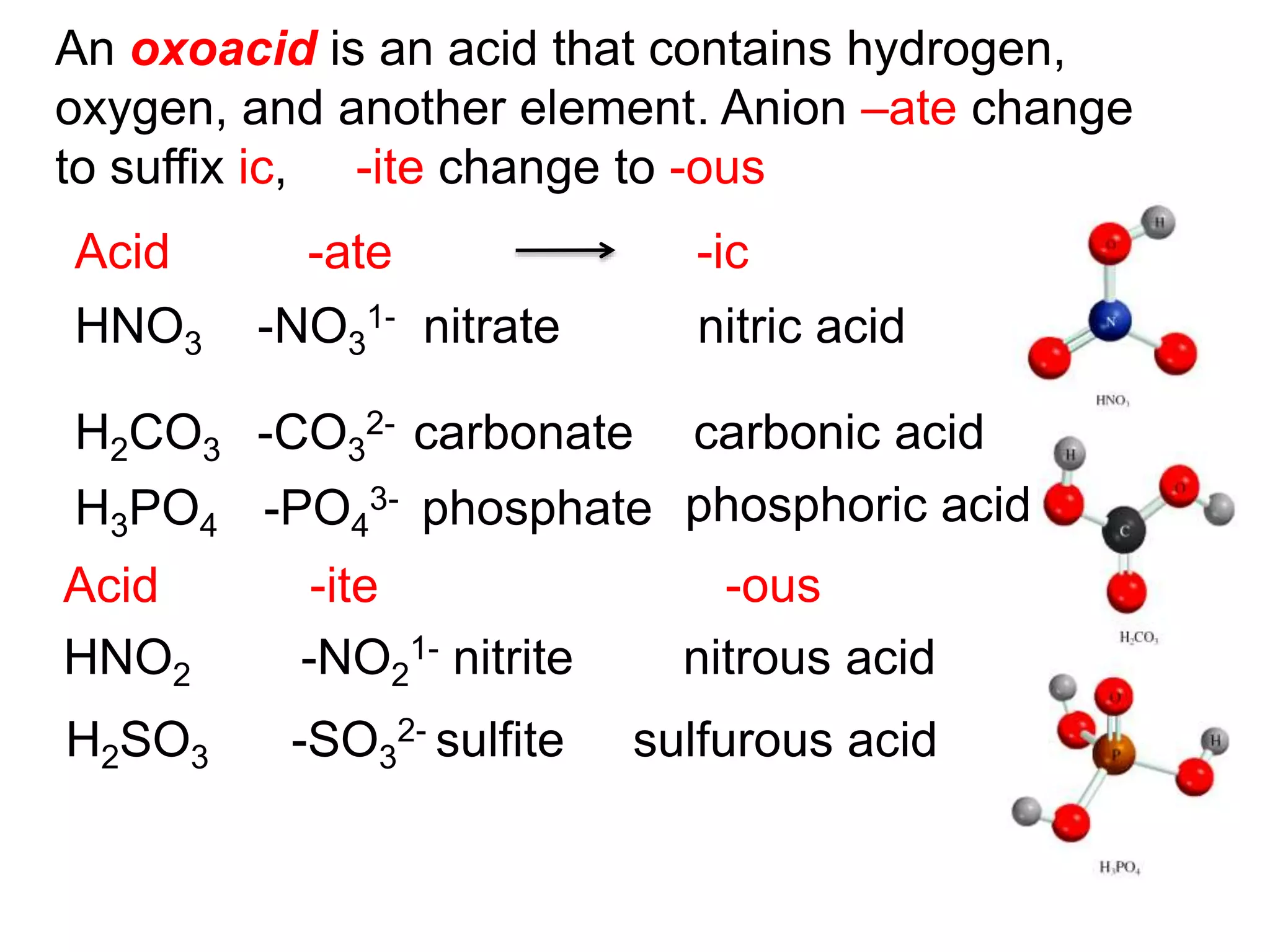
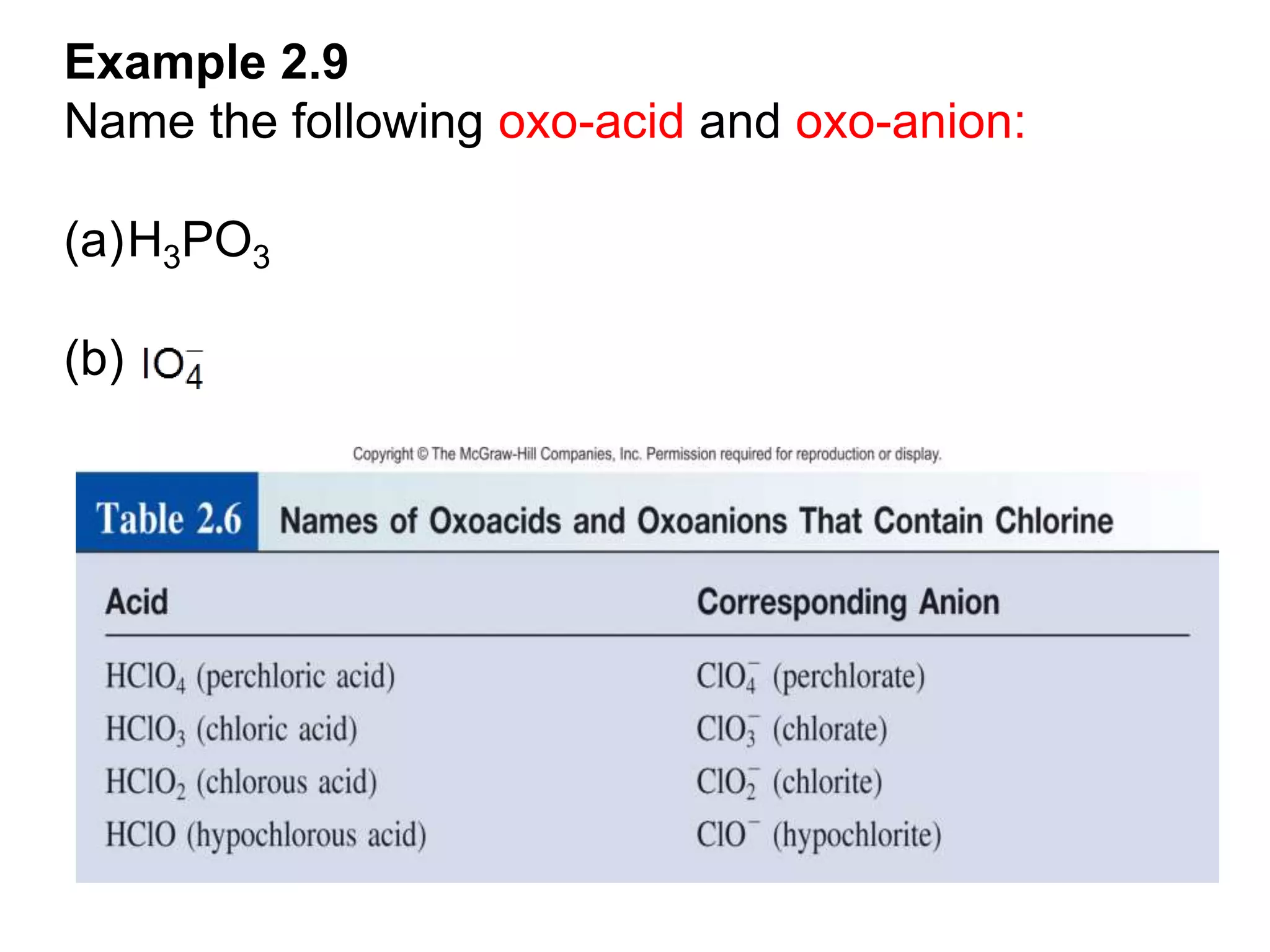
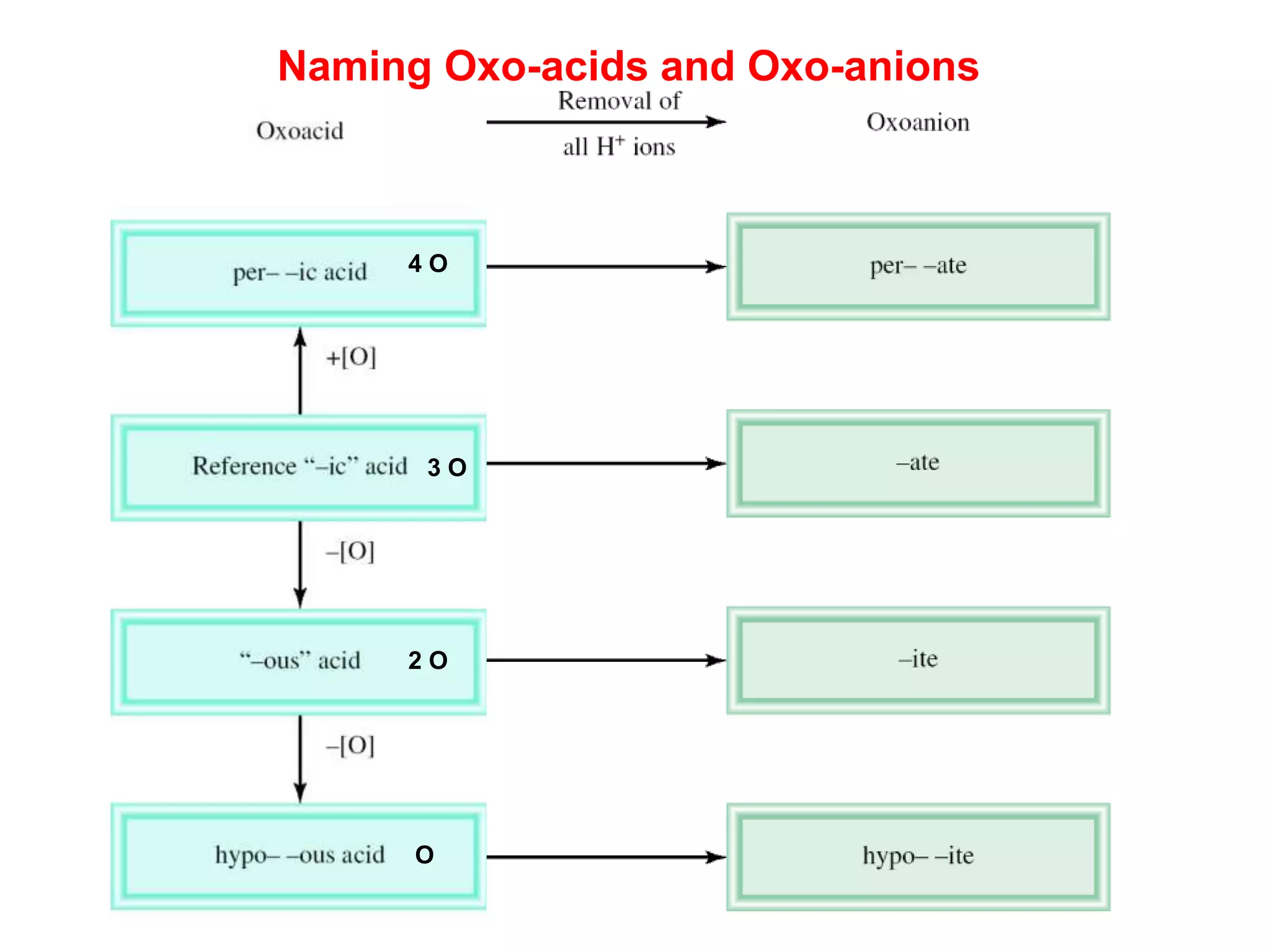
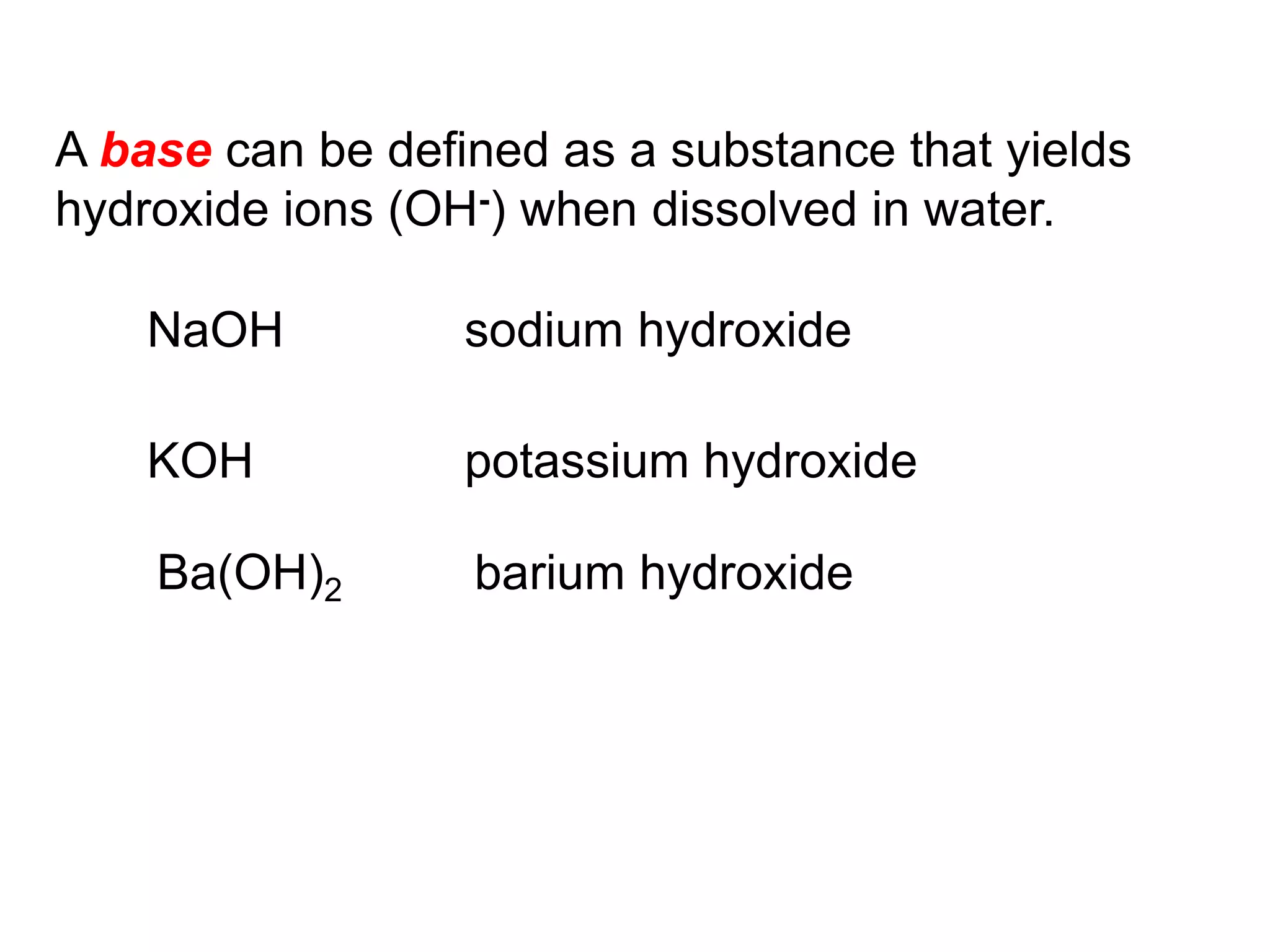
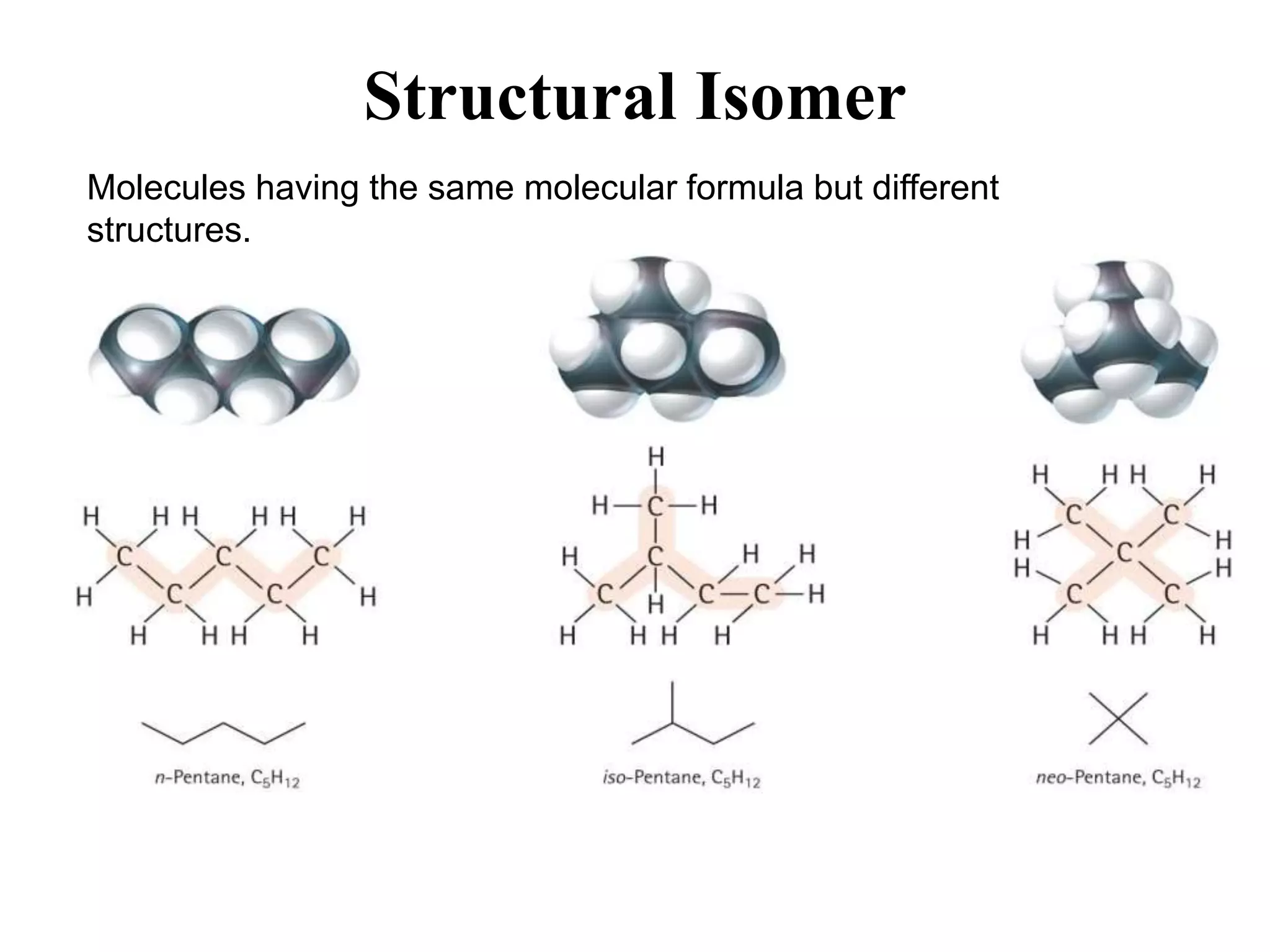
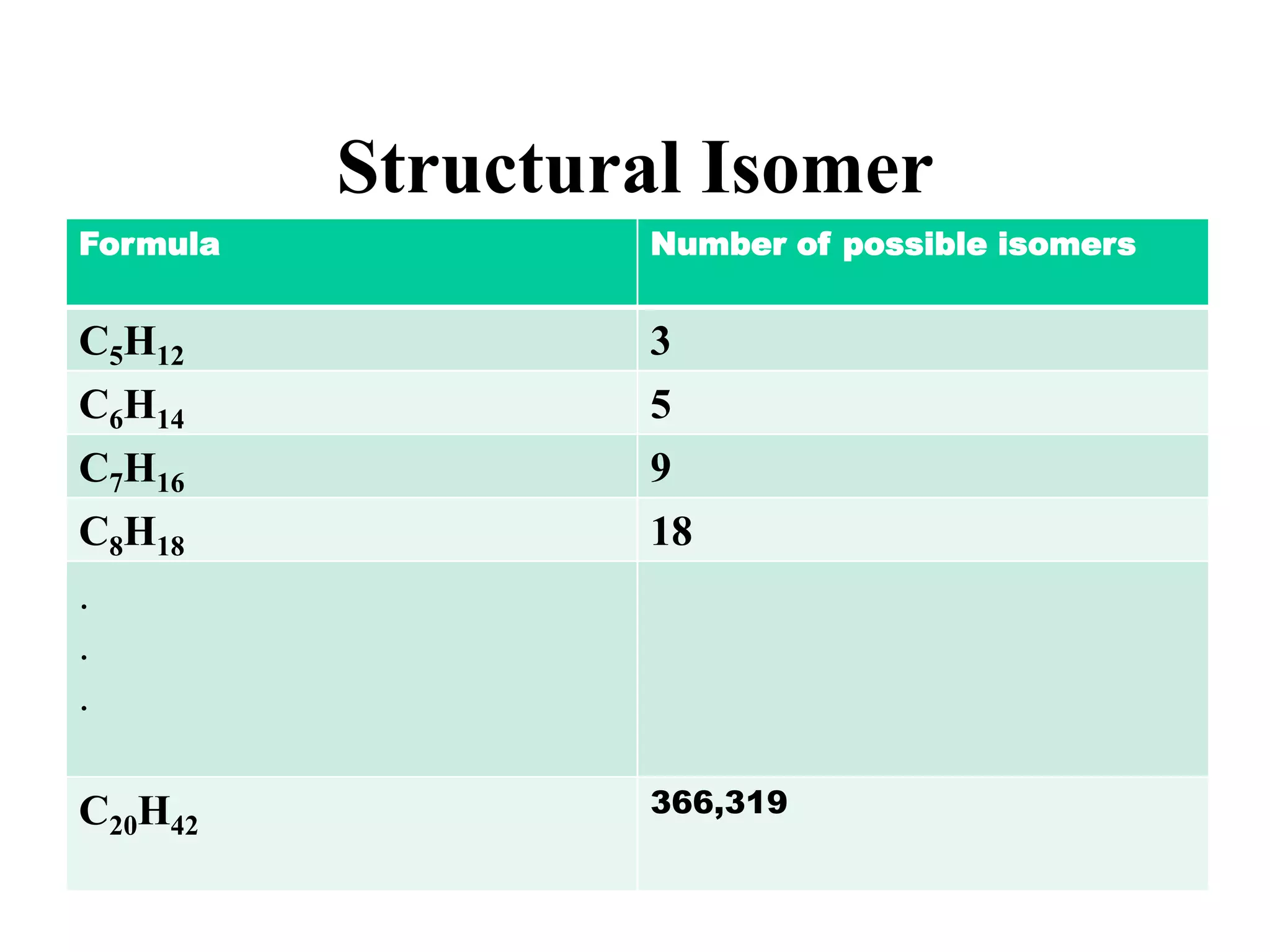
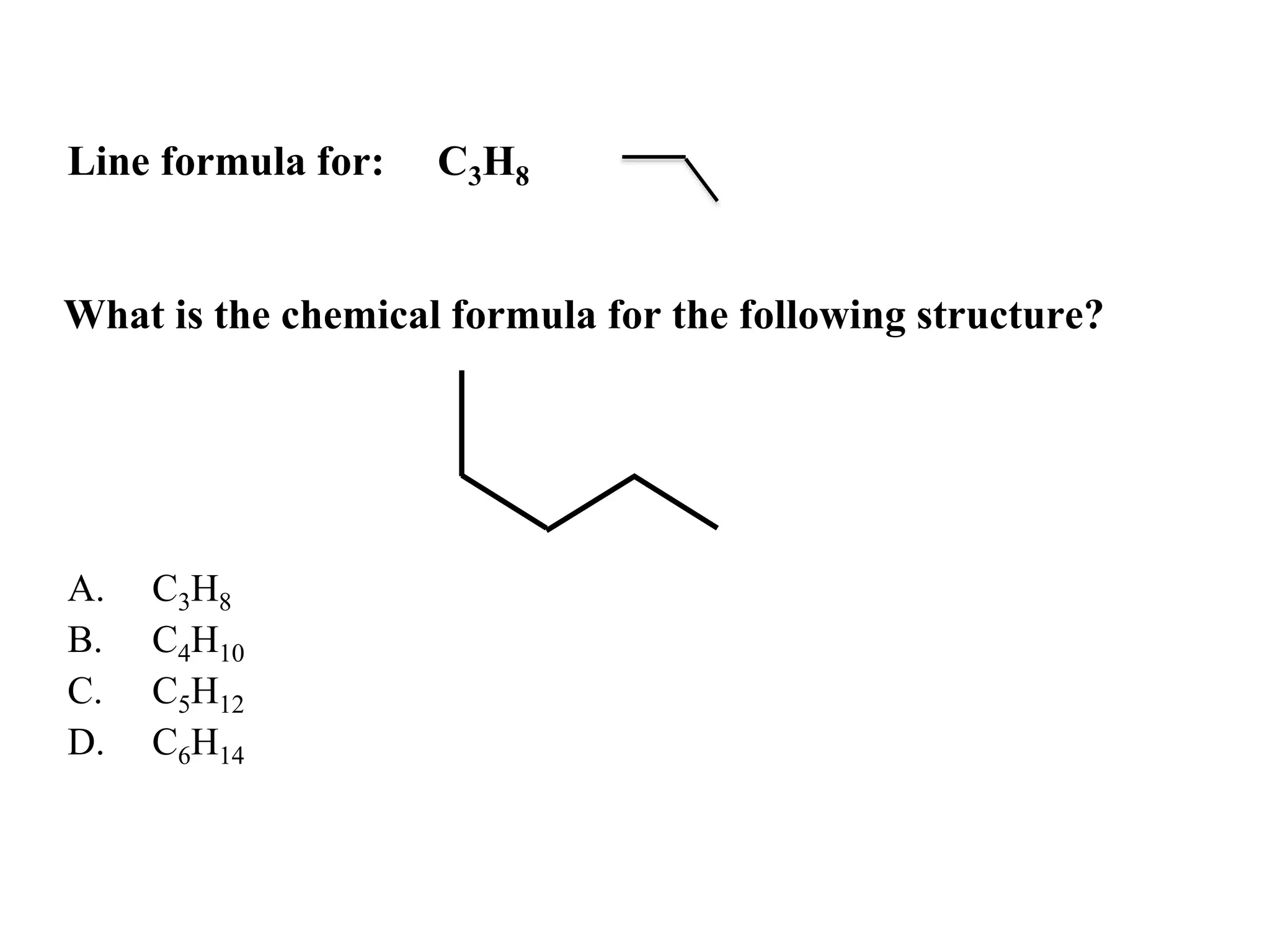
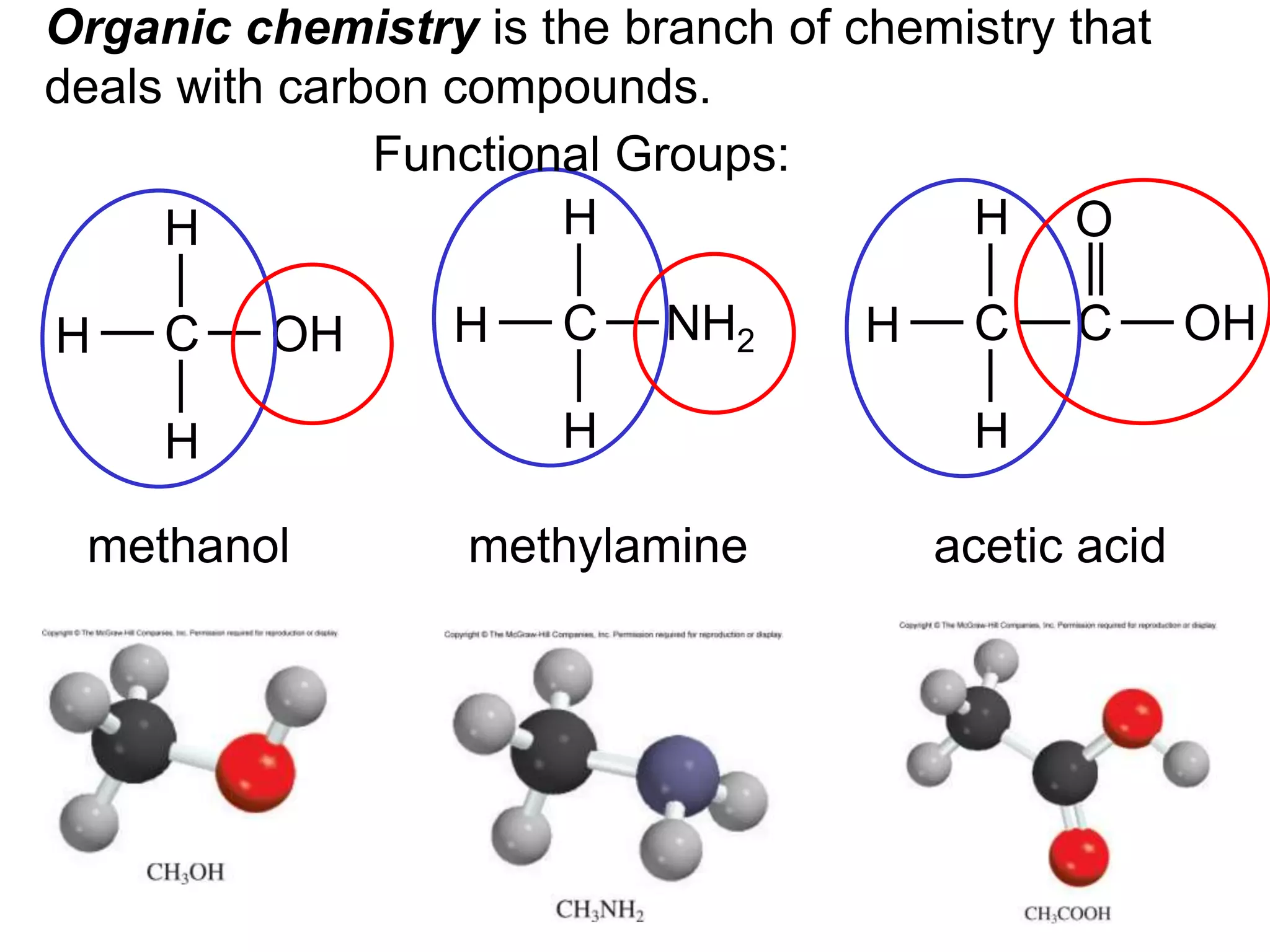
The document provides information about atoms, molecules, and ions. It discusses:
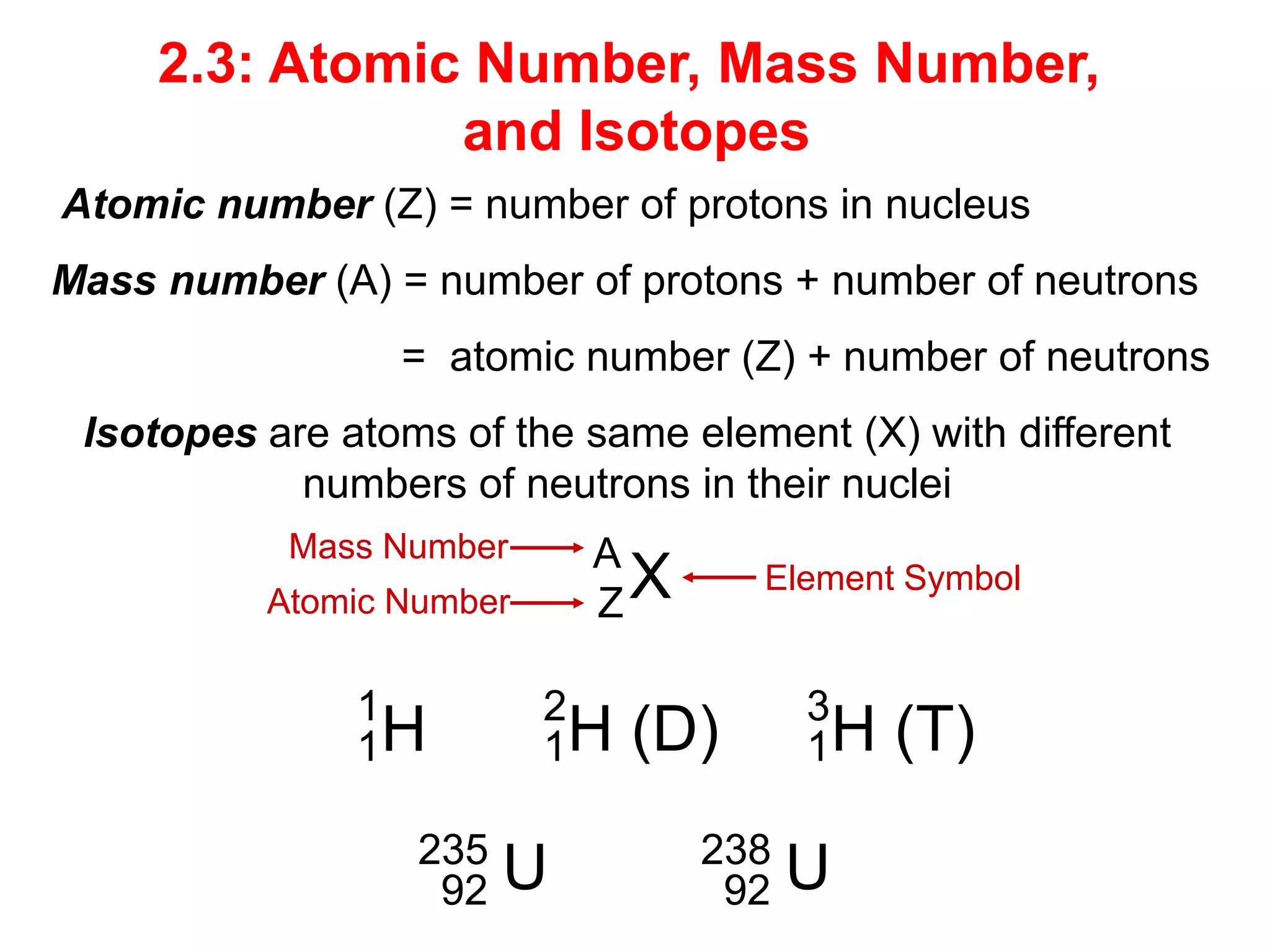
- Atomic number and mass number
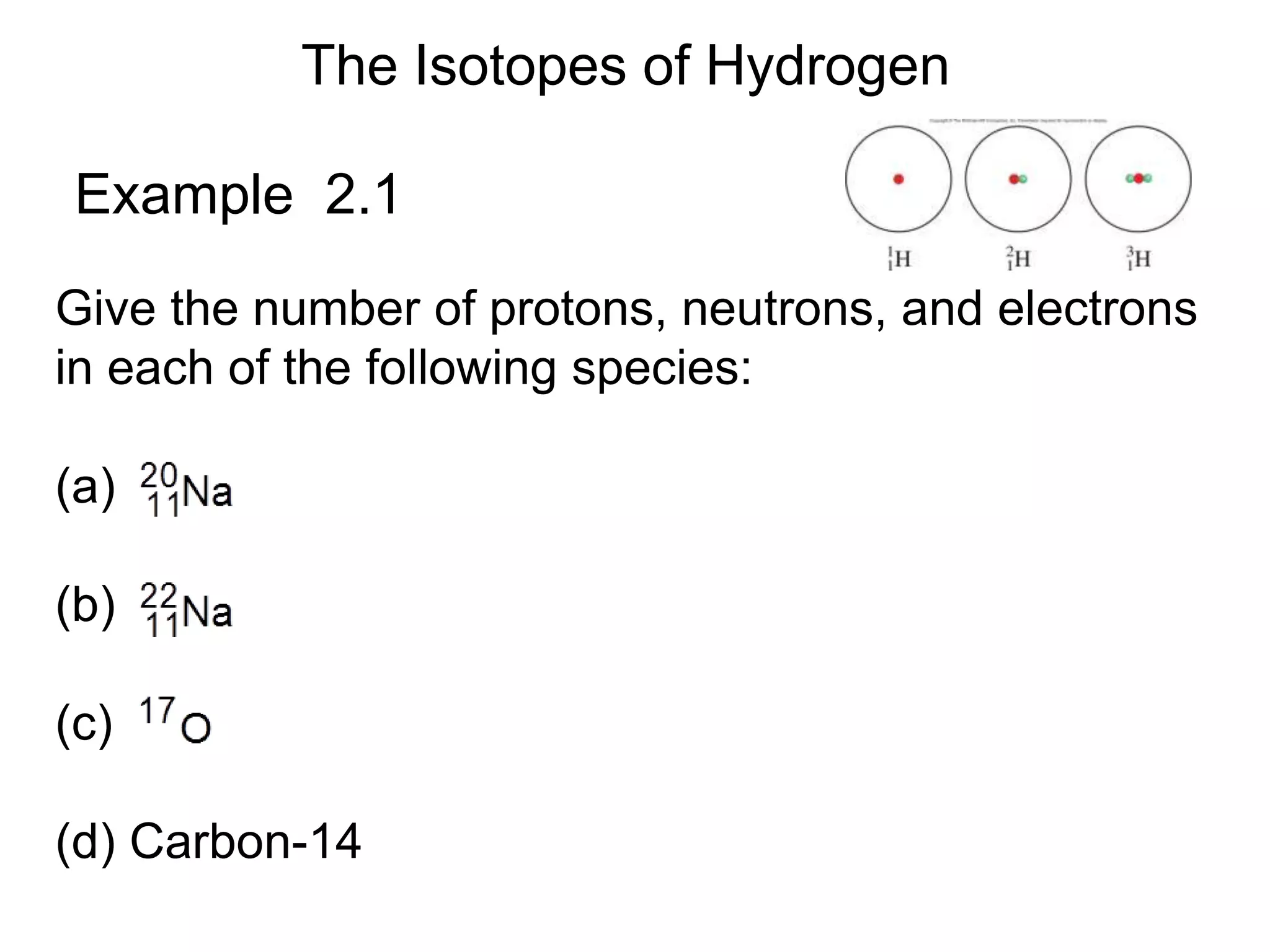
- Isotopes and examples of isotopes of hydrogen
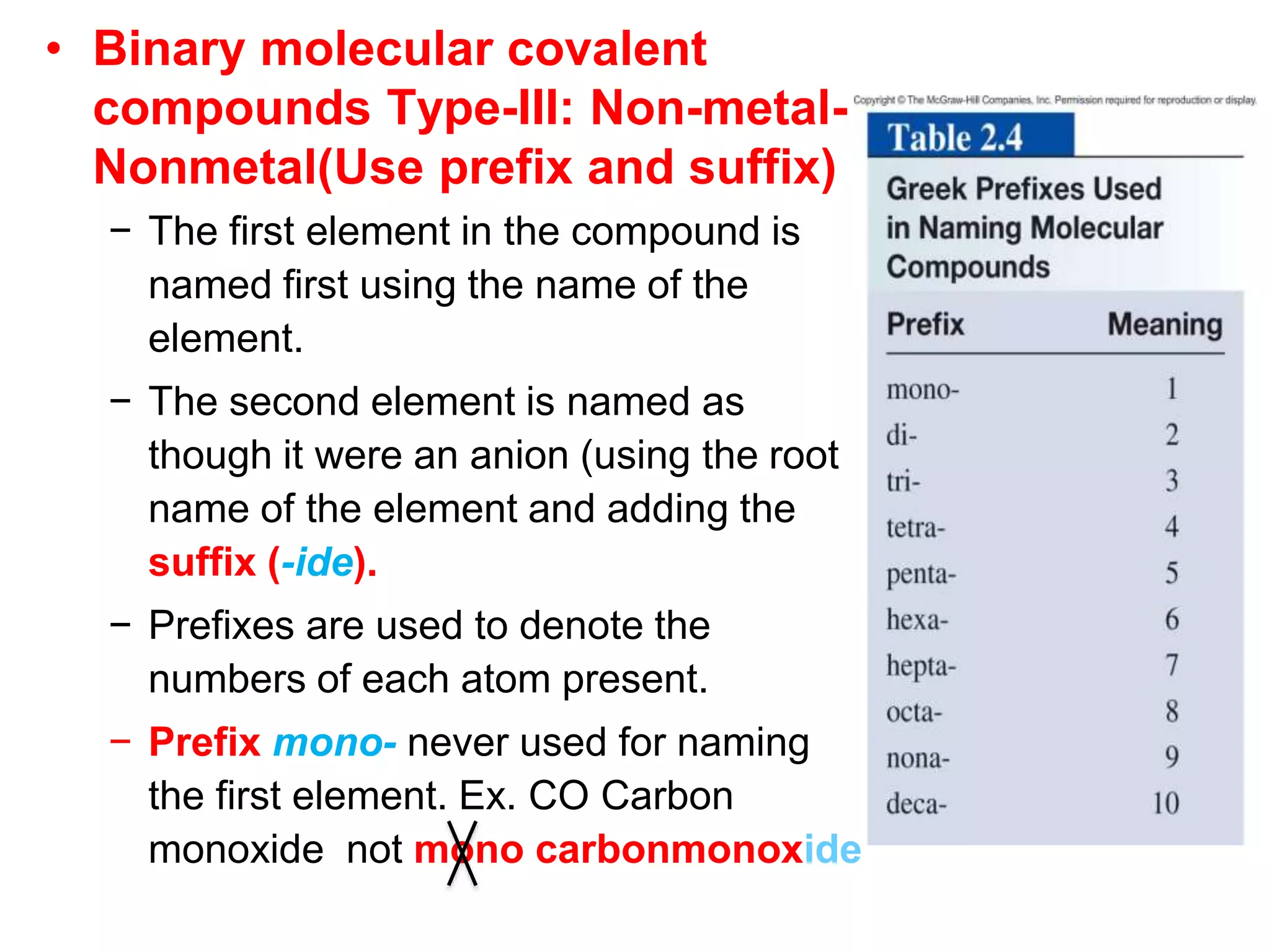
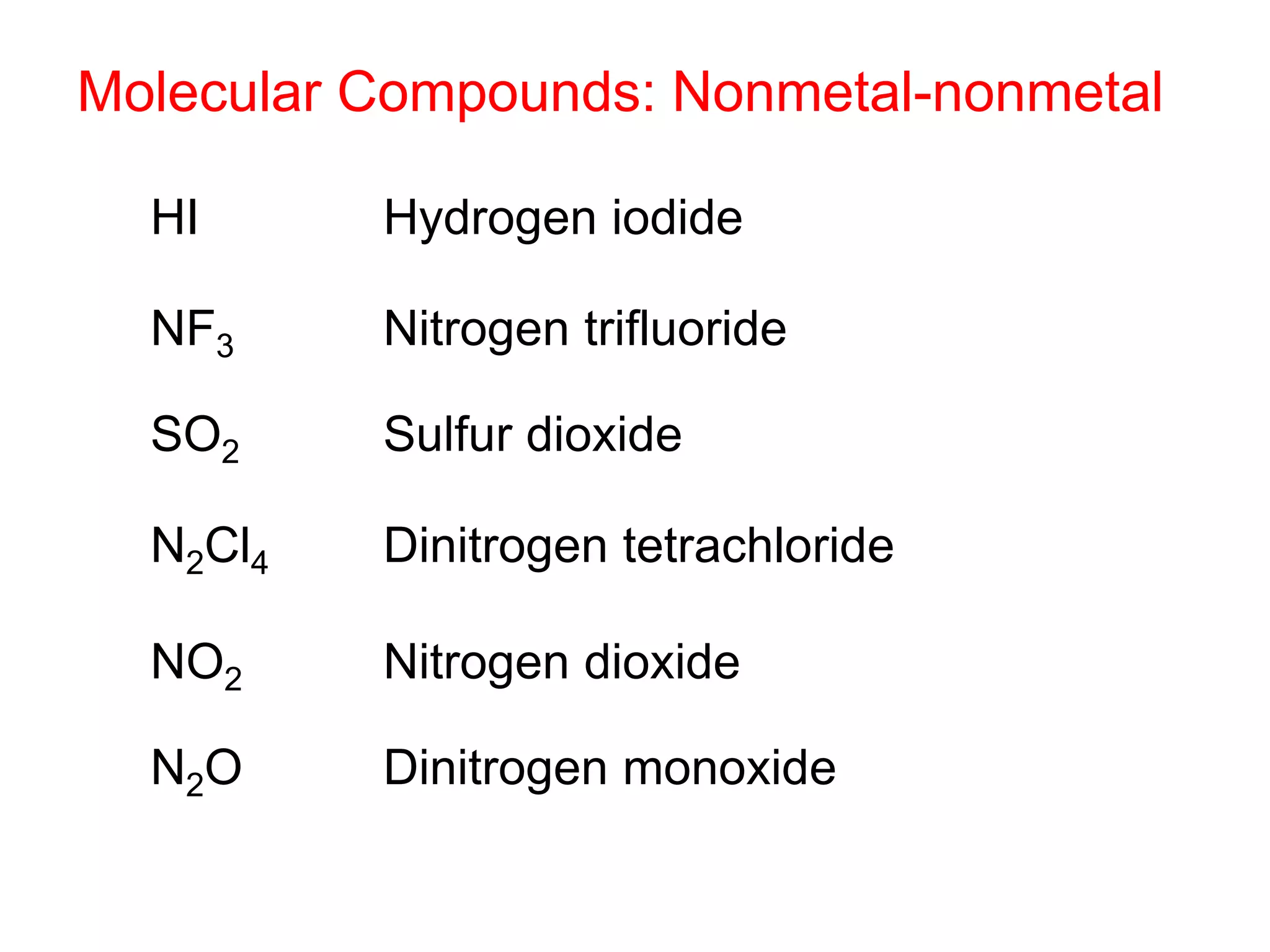
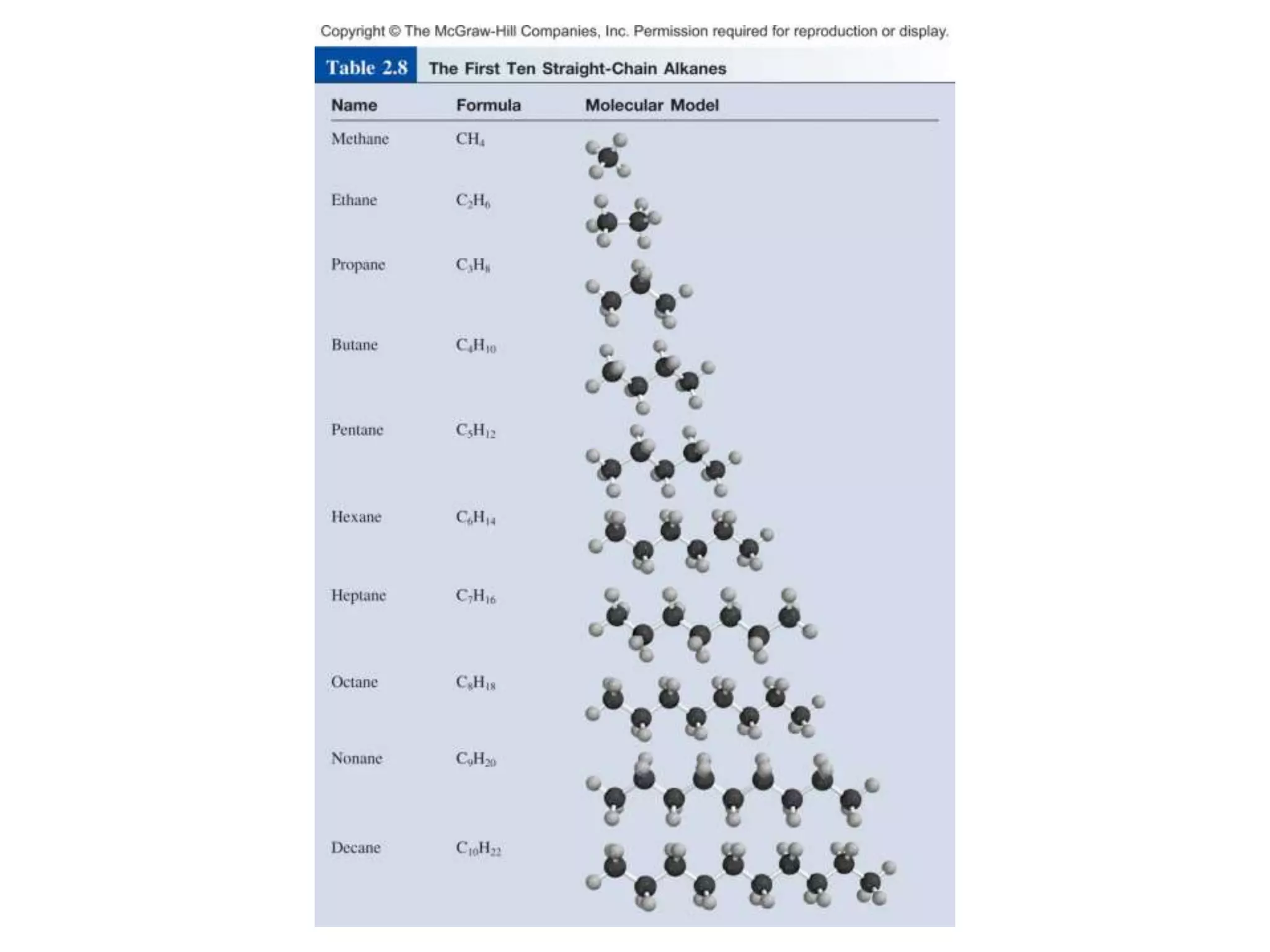
- Molecular and empirical formulas
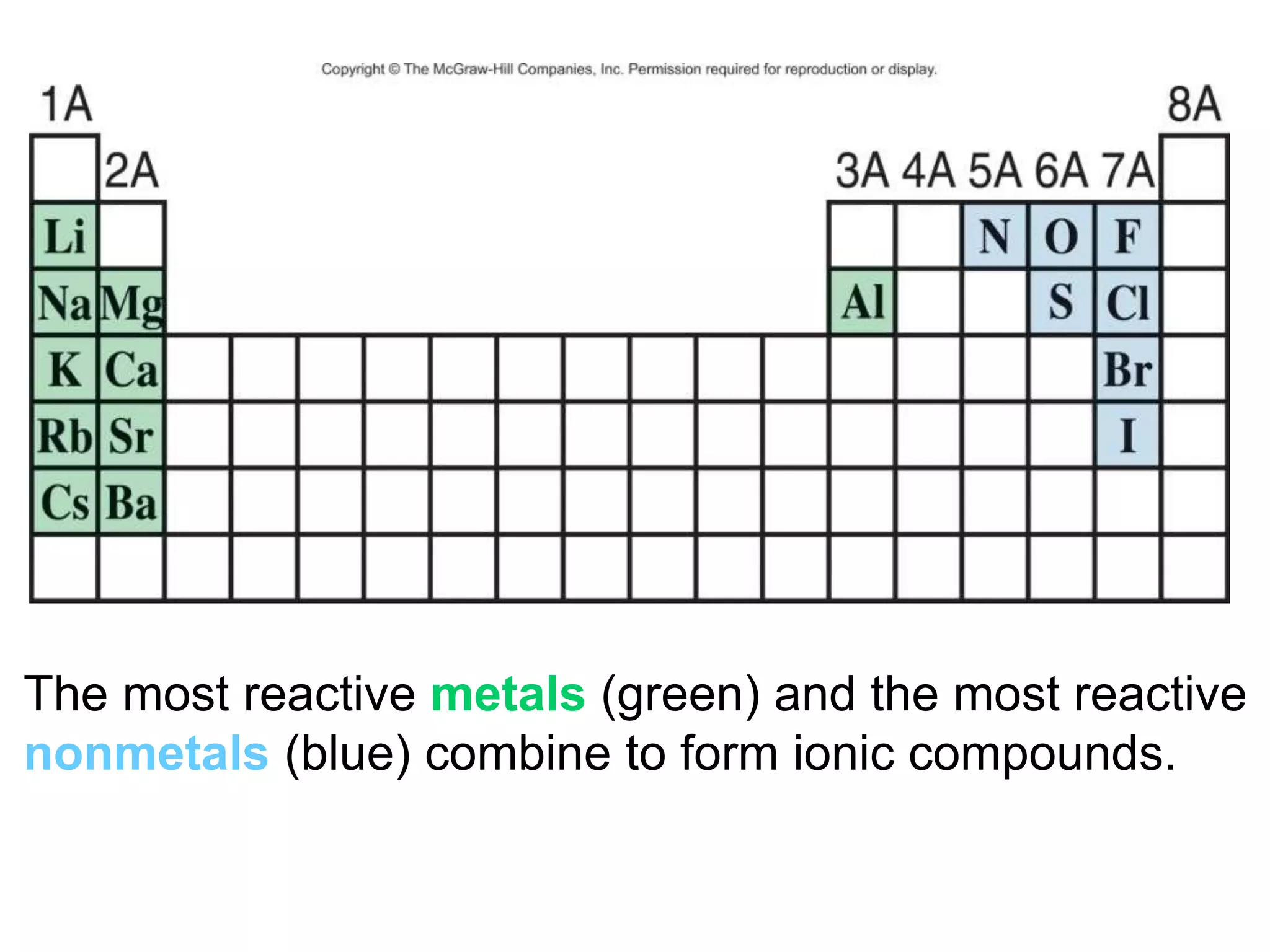
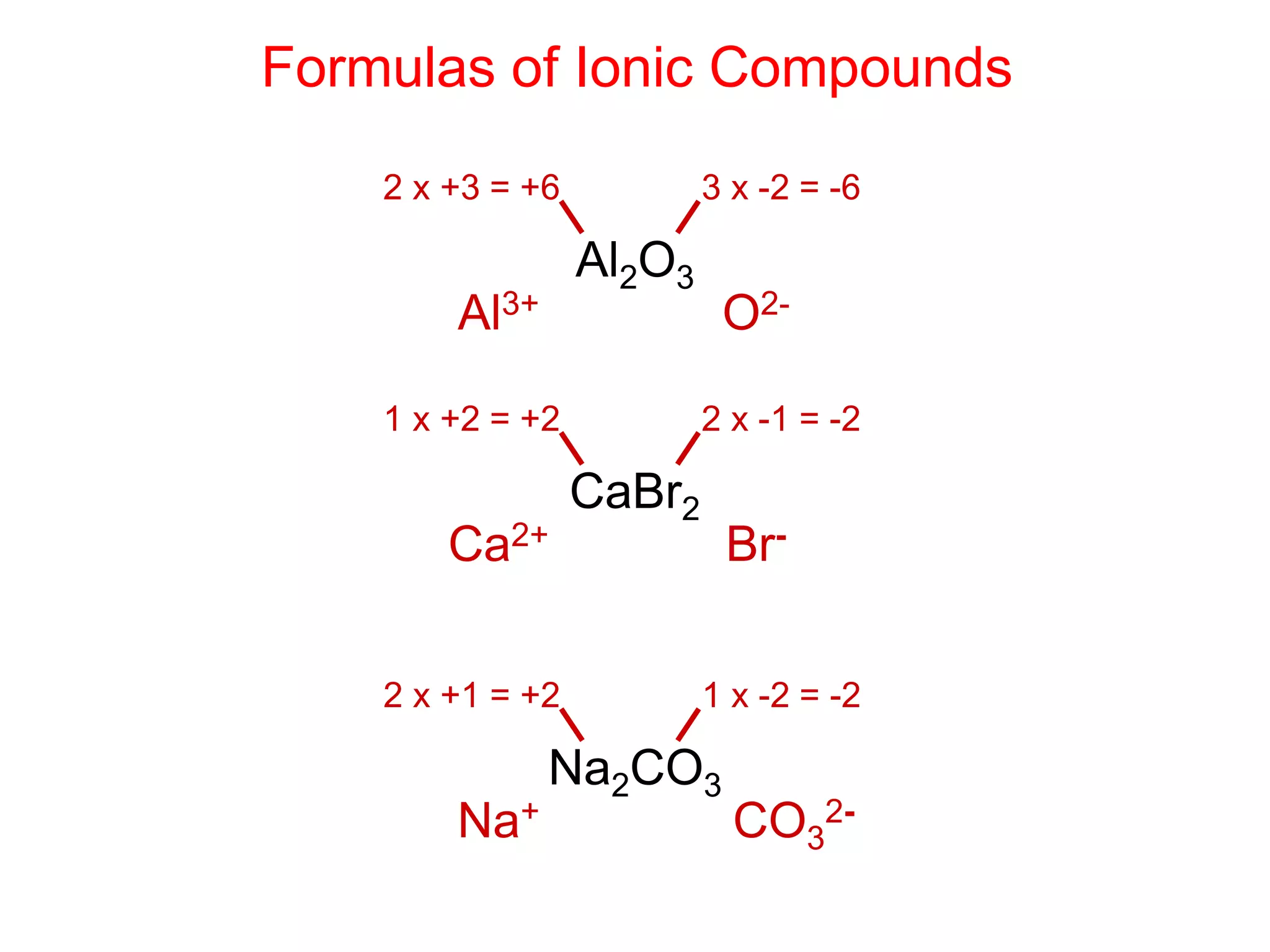
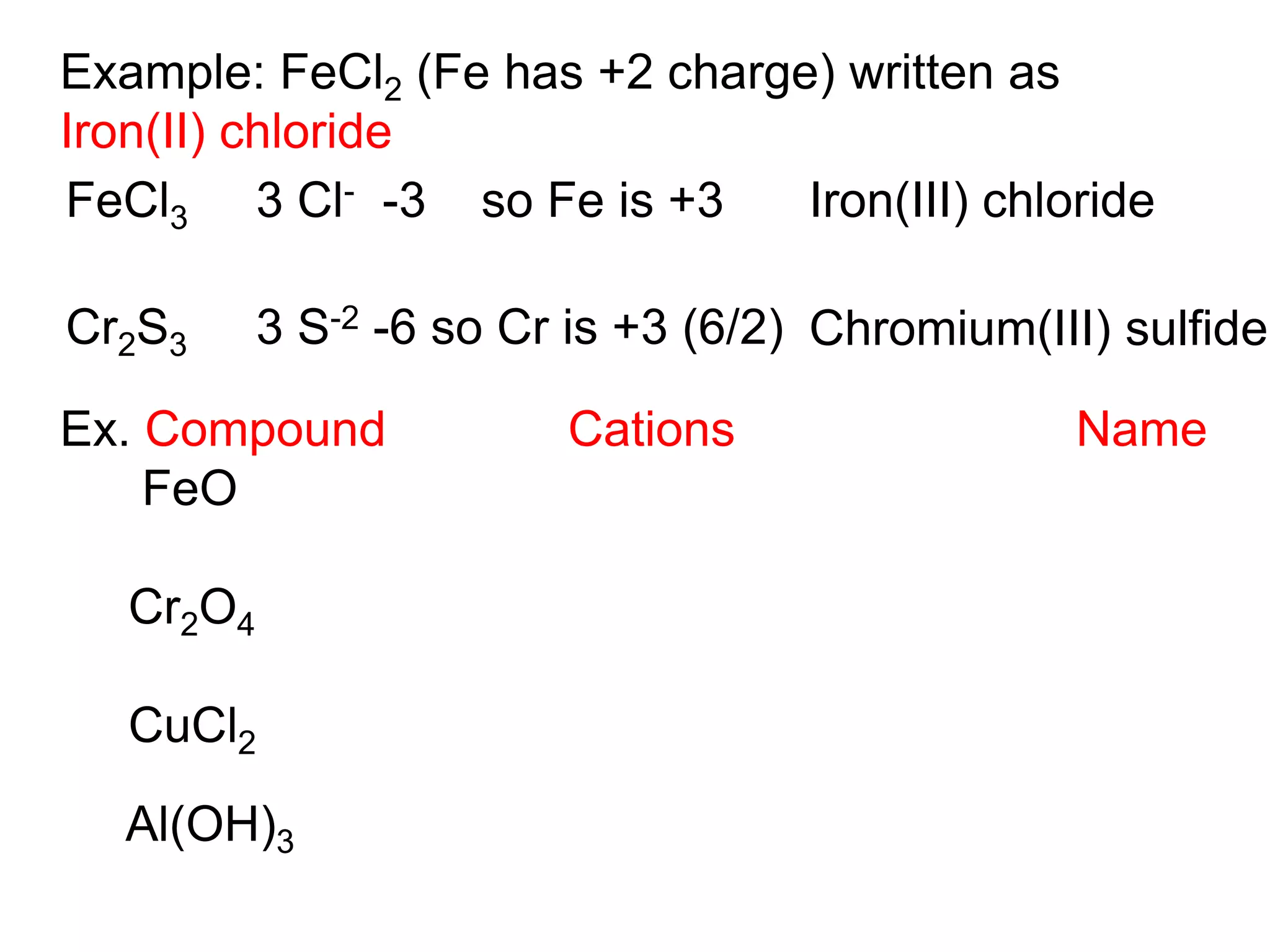
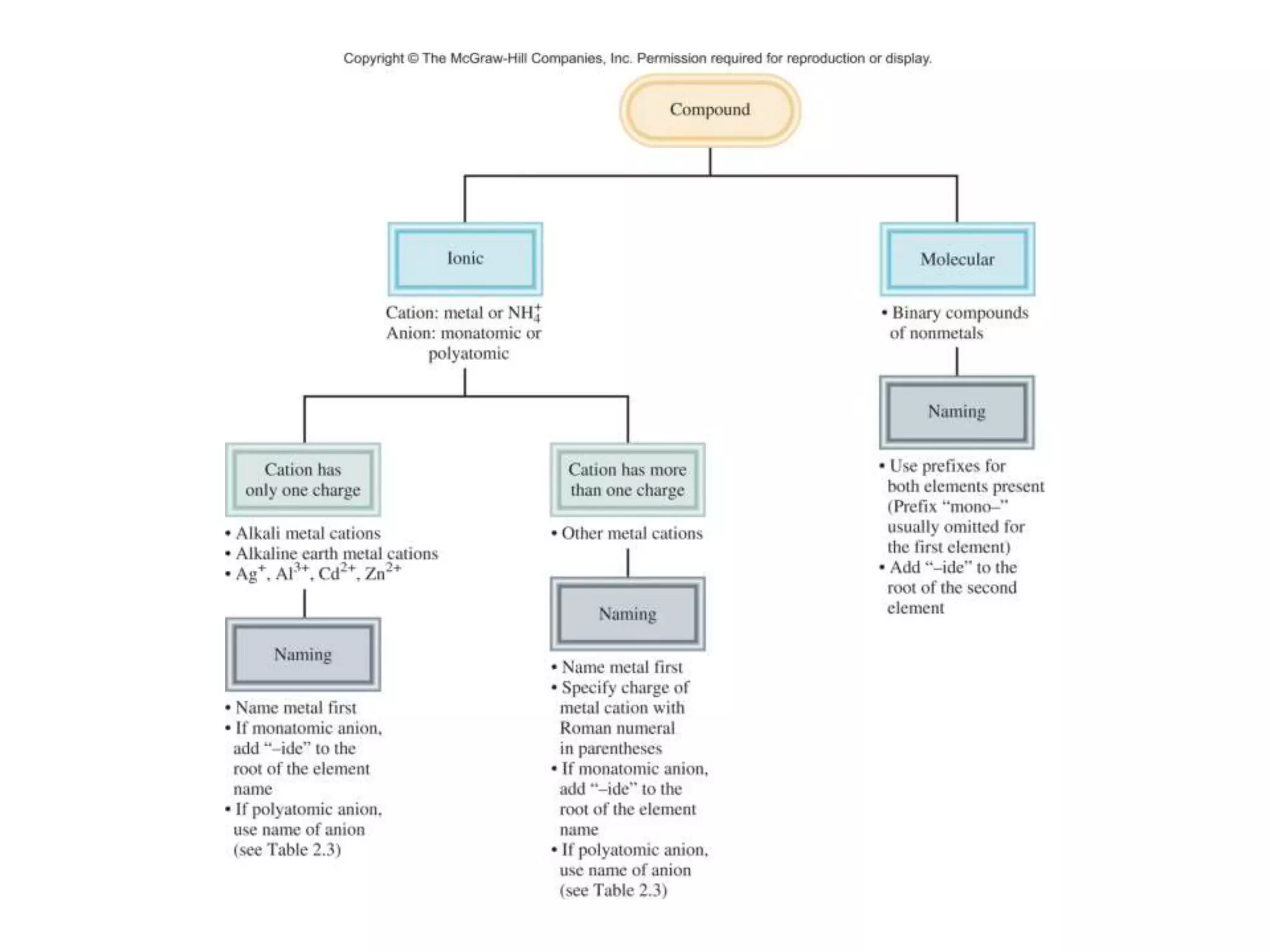
- Ionic compounds and how their formulas are determined
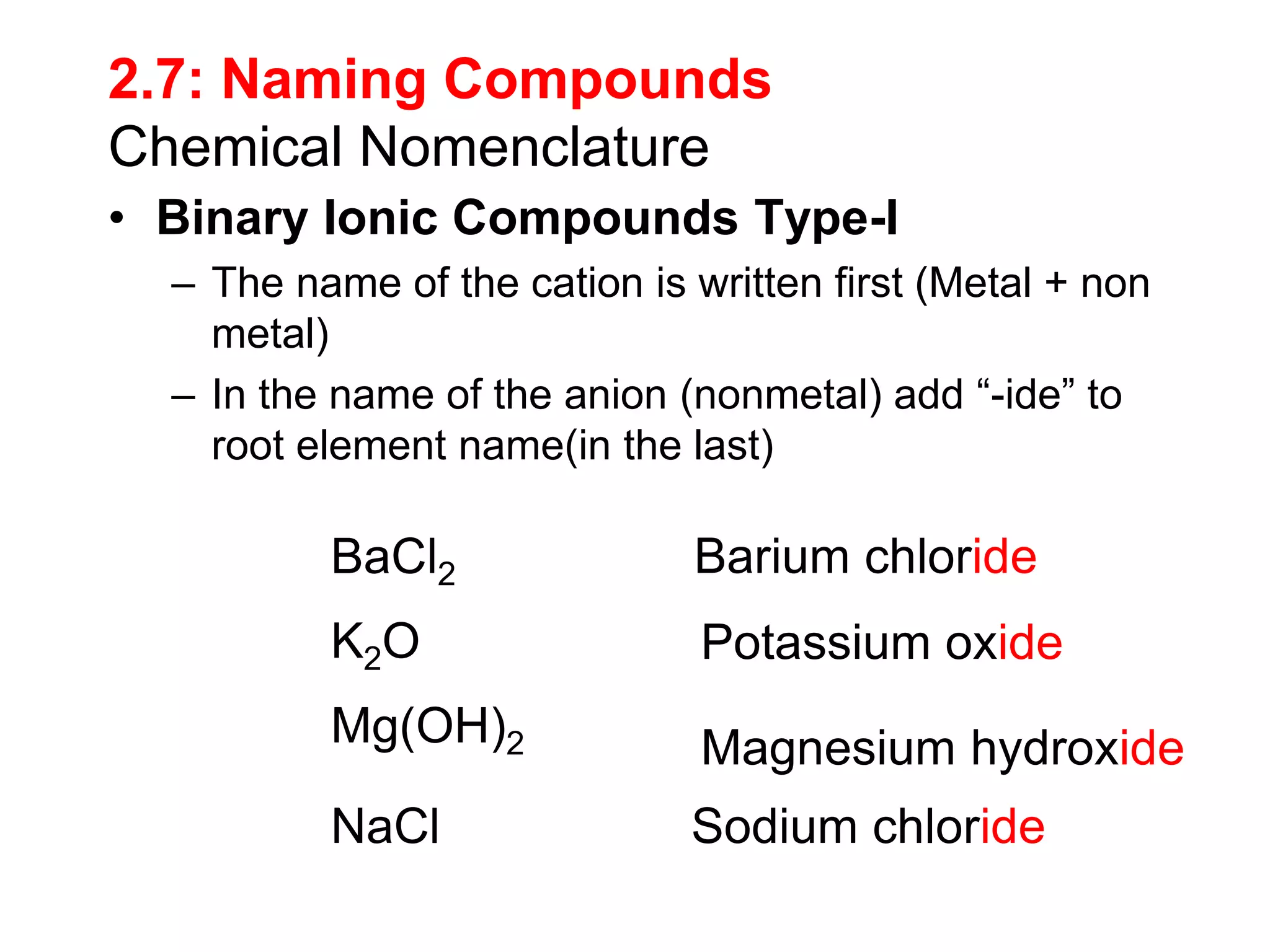
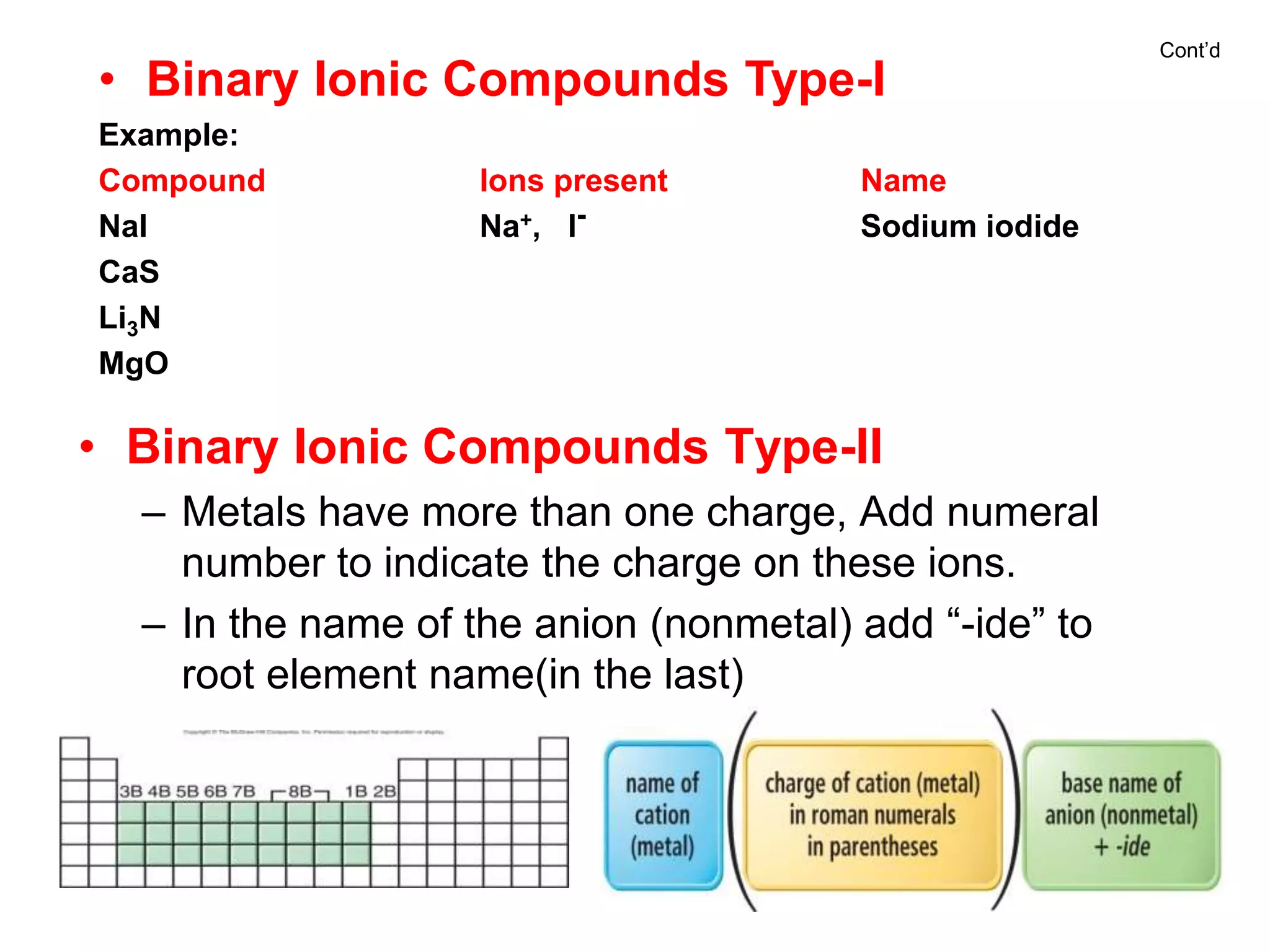
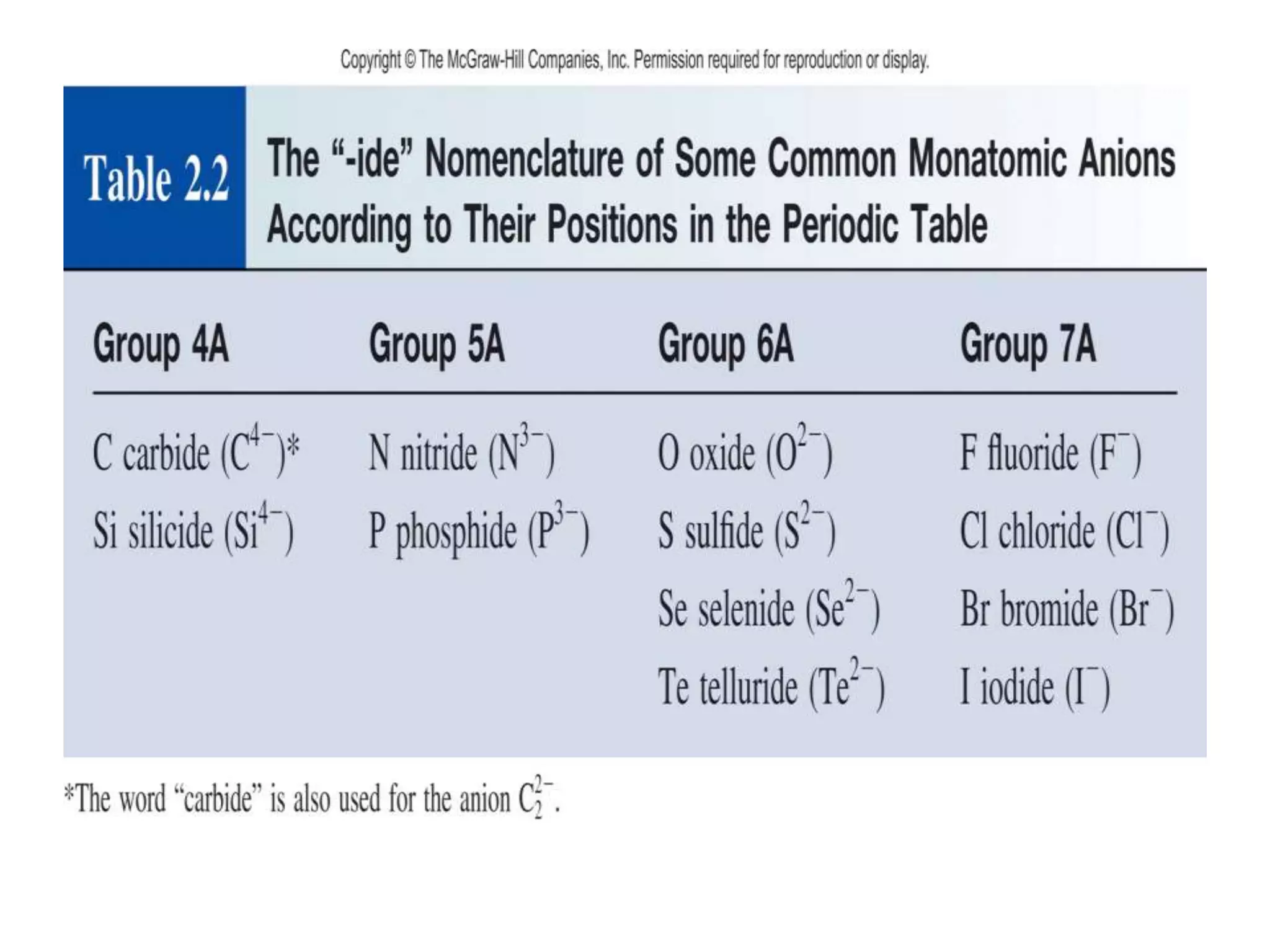
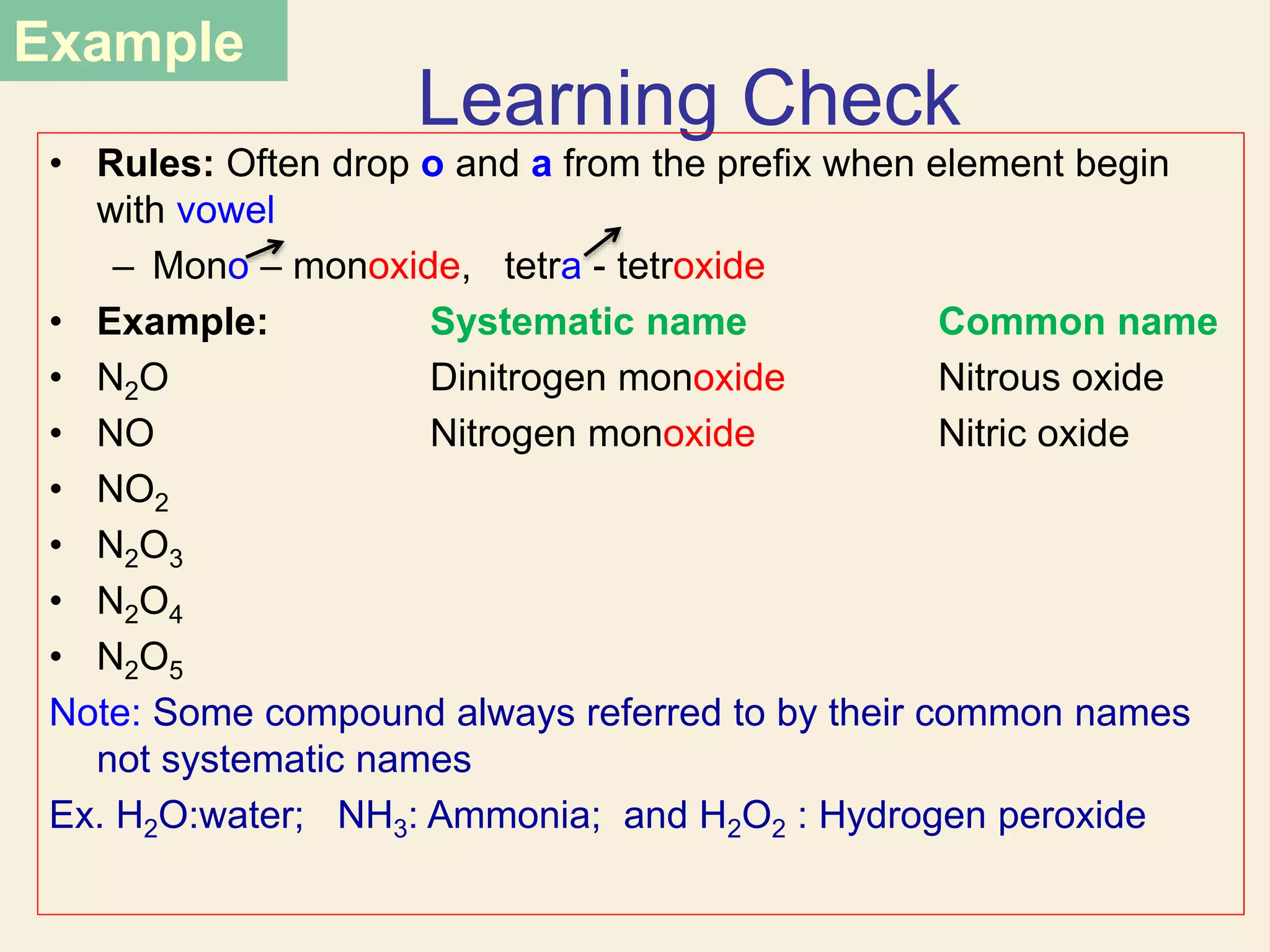
- Naming common compounds and ions
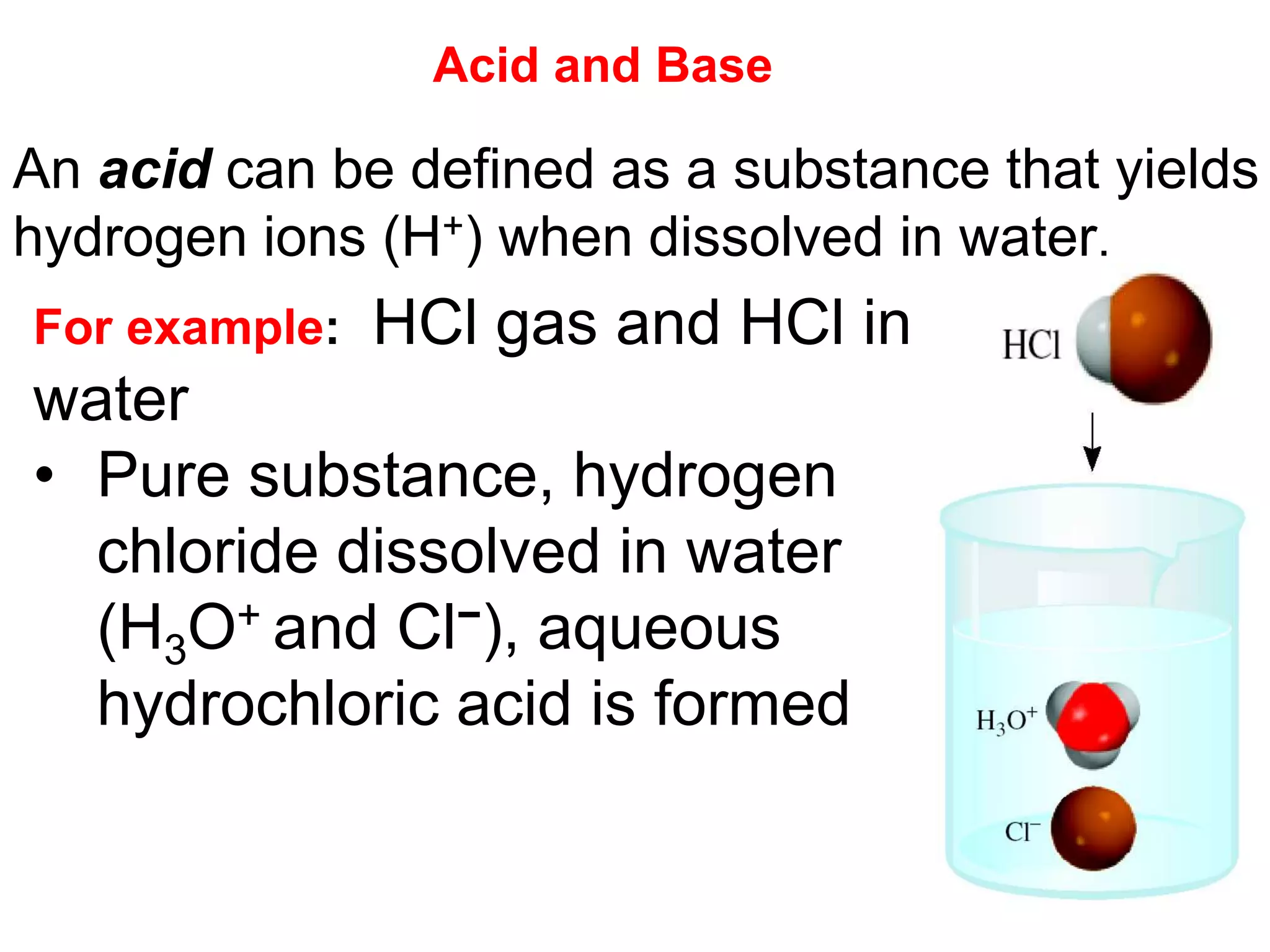
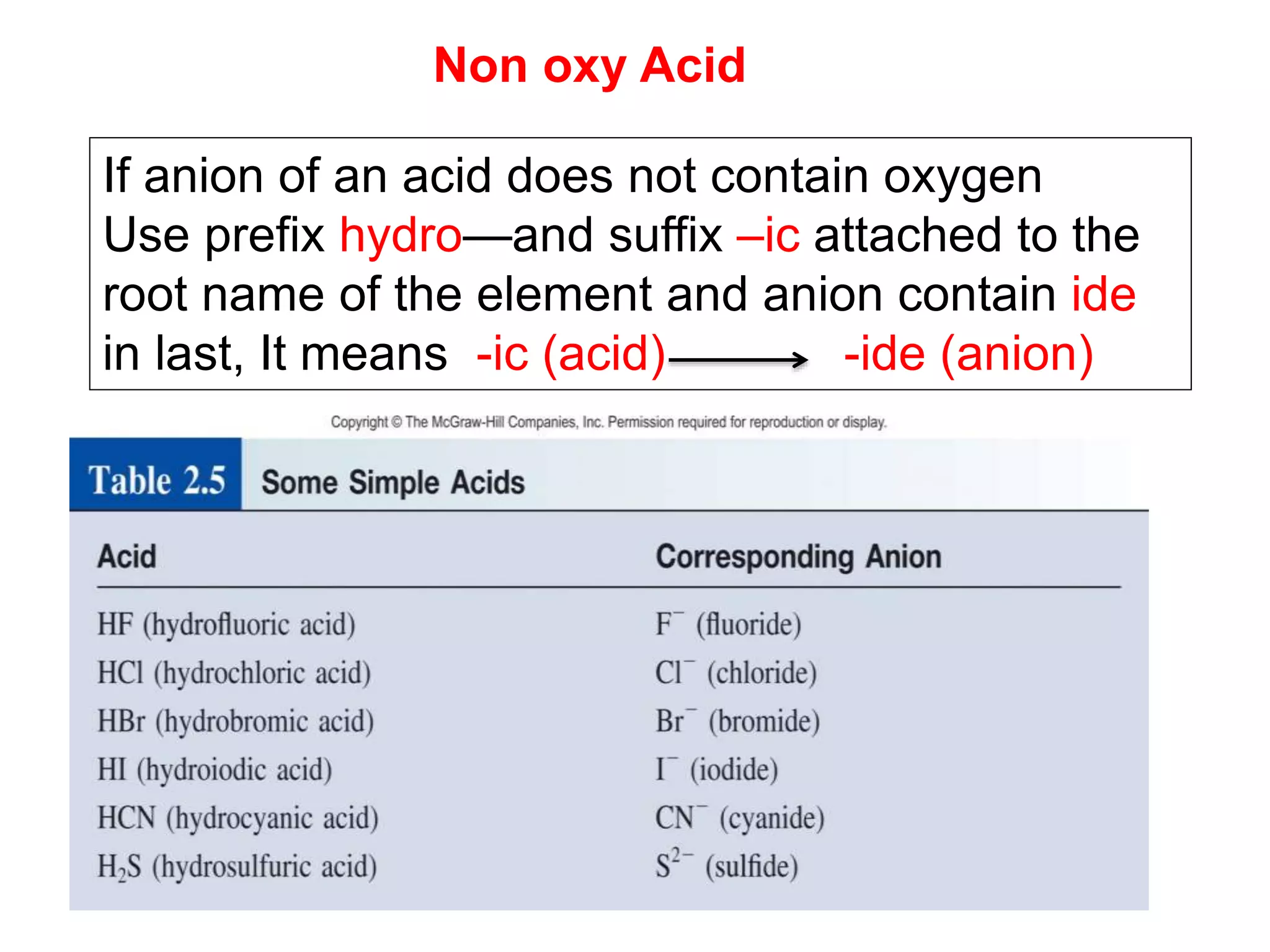
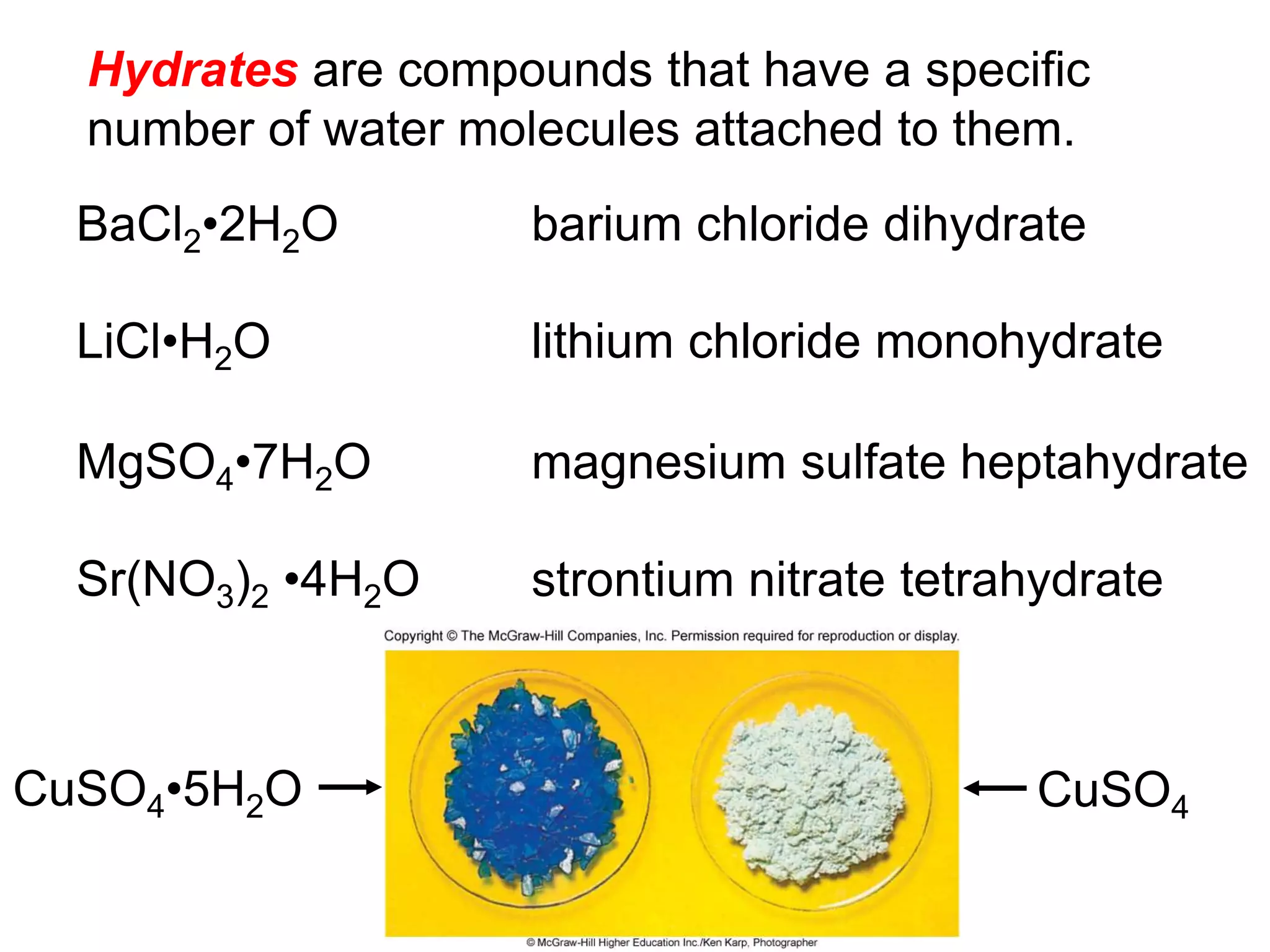
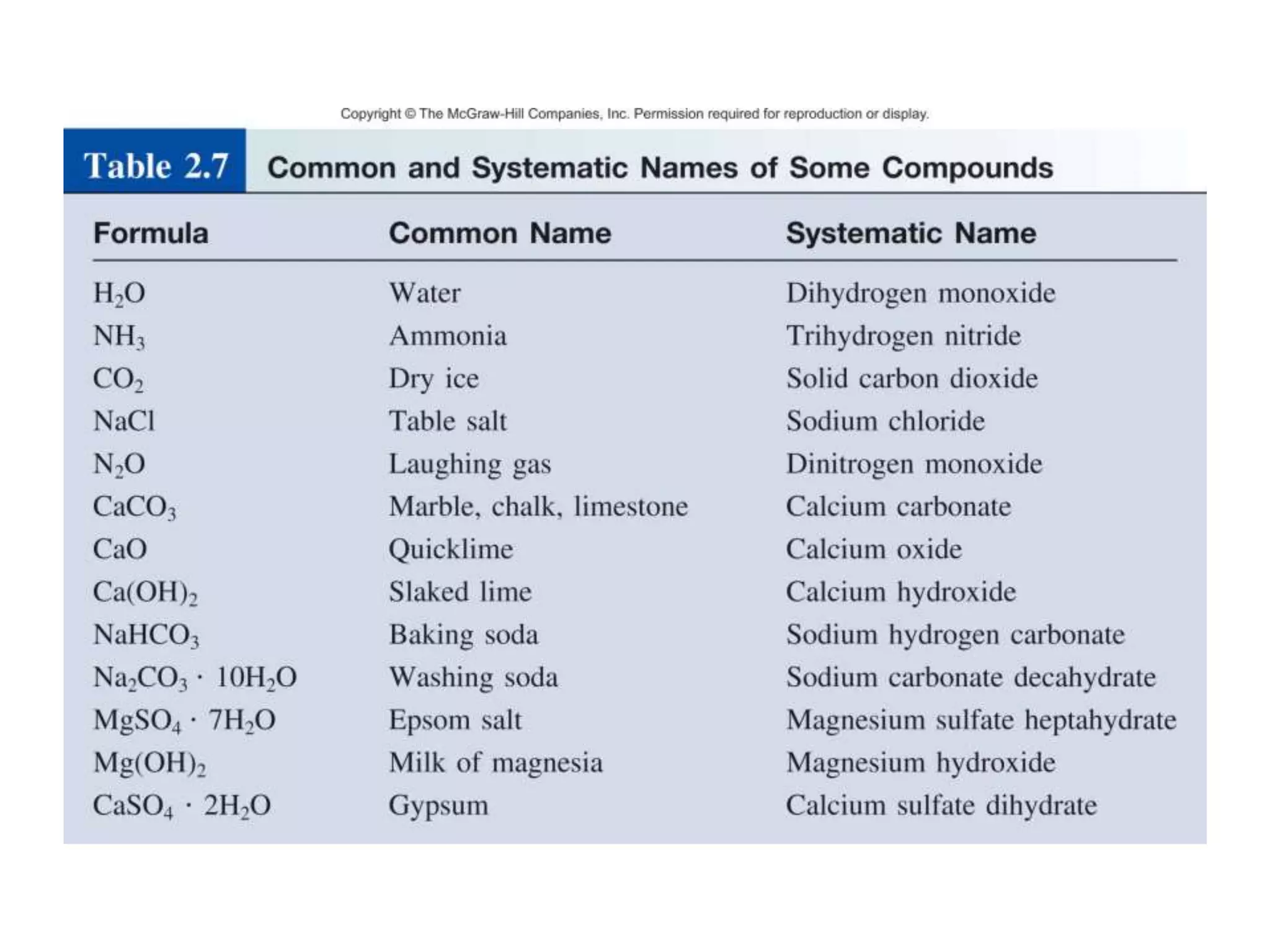
- Acids, bases, and naming acid anions and hydrate compounds