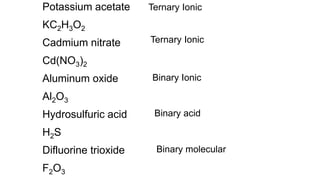
This document provides information on naming and writing formulas for different types of compounds:
- Binary molecular compounds contain two nonmetal elements joined by a covalent bond. Prefixes are used to name them and indicate the number of atoms in the formula.
- Binary acids contain hydrogen and one nonmetal element. They follow a consistent naming pattern of "hydro-" plus the nonmetal root plus "-ic acid".
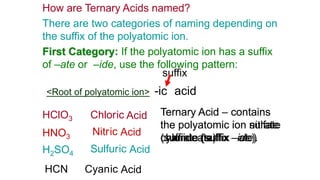
- Ternary acids contain hydrogen and a polyatomic ion. Their names indicate whether the polyatomic ion ends in "-ate", "-ite" or another suffix. Formulas are written by balancing hydrogen's +1 charge with the charges of the other elements/ions.