This document discusses several methods for isolating and separating proteins, including:
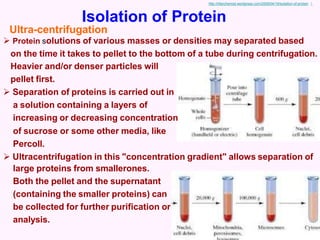
- Ultracentrifugation, which separates proteins based on mass and density over time in a centrifuge.
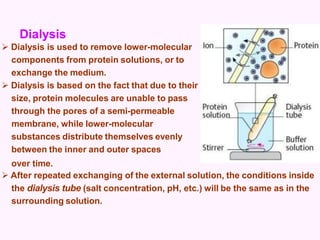
- Dialysis, which removes smaller molecules from a protein solution through a semi-permeable membrane.
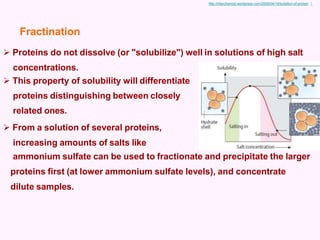
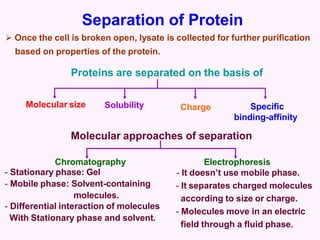
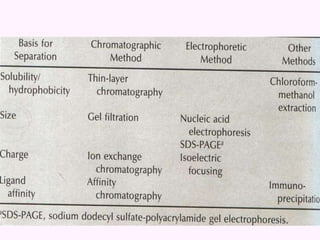
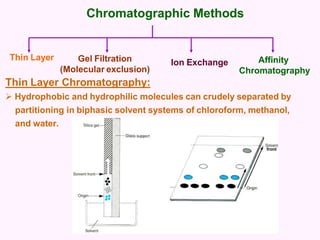
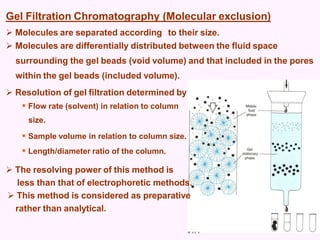
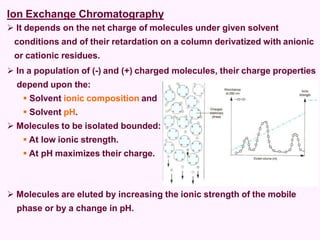
- Chromatography techniques like gel filtration, ion exchange, and affinity chromatography separate proteins based on size, charge, and binding affinity to separate mixtures.
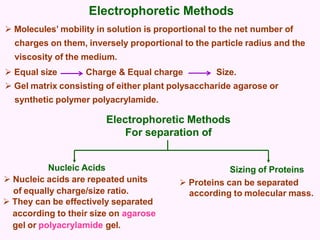
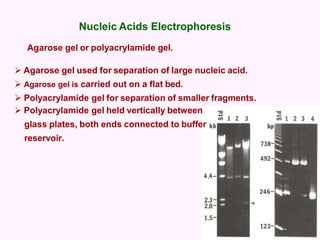
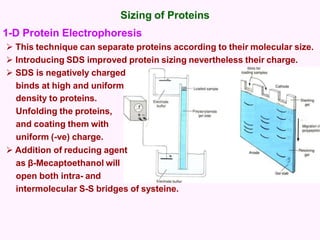
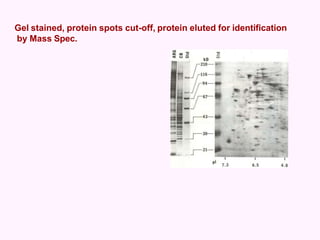
- Electrophoresis techniques like agarose/polyacrylamide gel electrophoresis and 2D electrophoresis separate proteins based on size and isoelectric point to further purify samples.