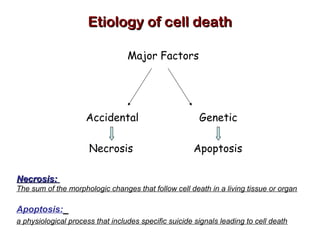
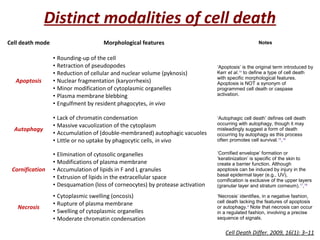
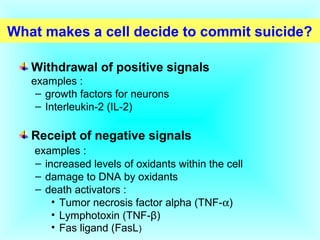
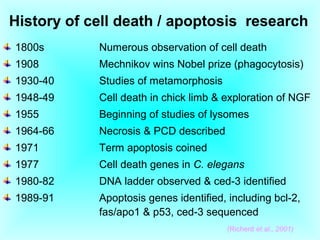
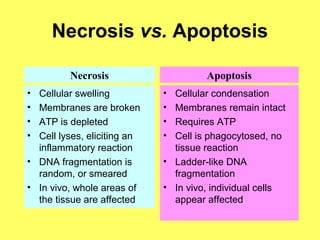
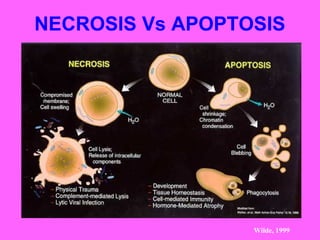
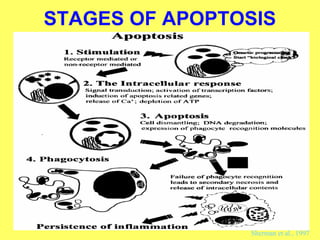
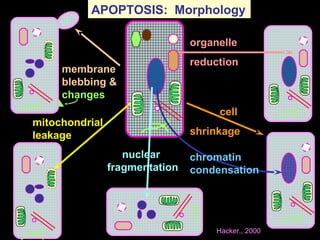
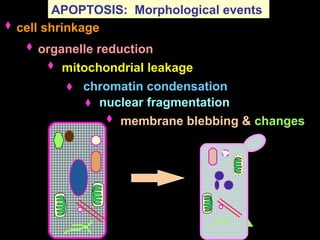
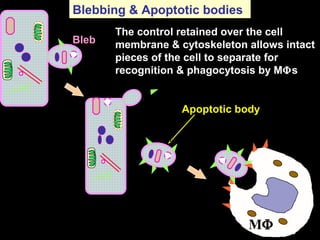
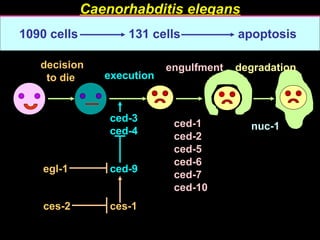
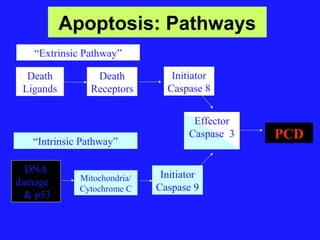
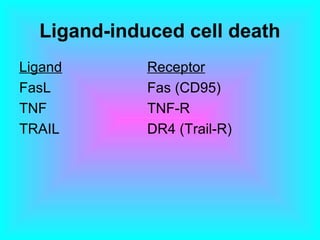
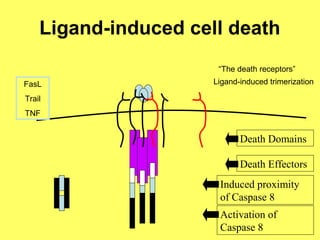
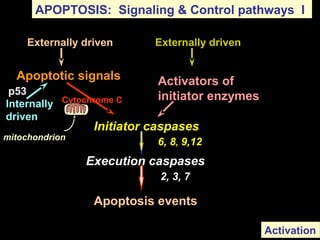
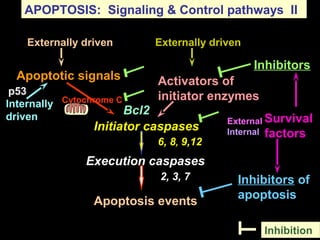
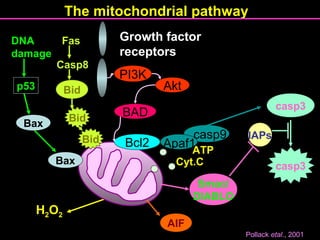
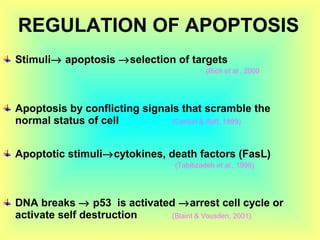
The document discusses the mechanisms and importance of different types of cell death, specifically apoptosis, necrosis, and autophagy. It highlights the physiological roles of apoptosis in development, maintenance of organism health, and its implications in diseases such as cancer and neurodegenerative disorders. Additionally, it outlines the regulatory pathways and signals involved in triggering cell death processes.