Embed presentation
Downloaded 29 times



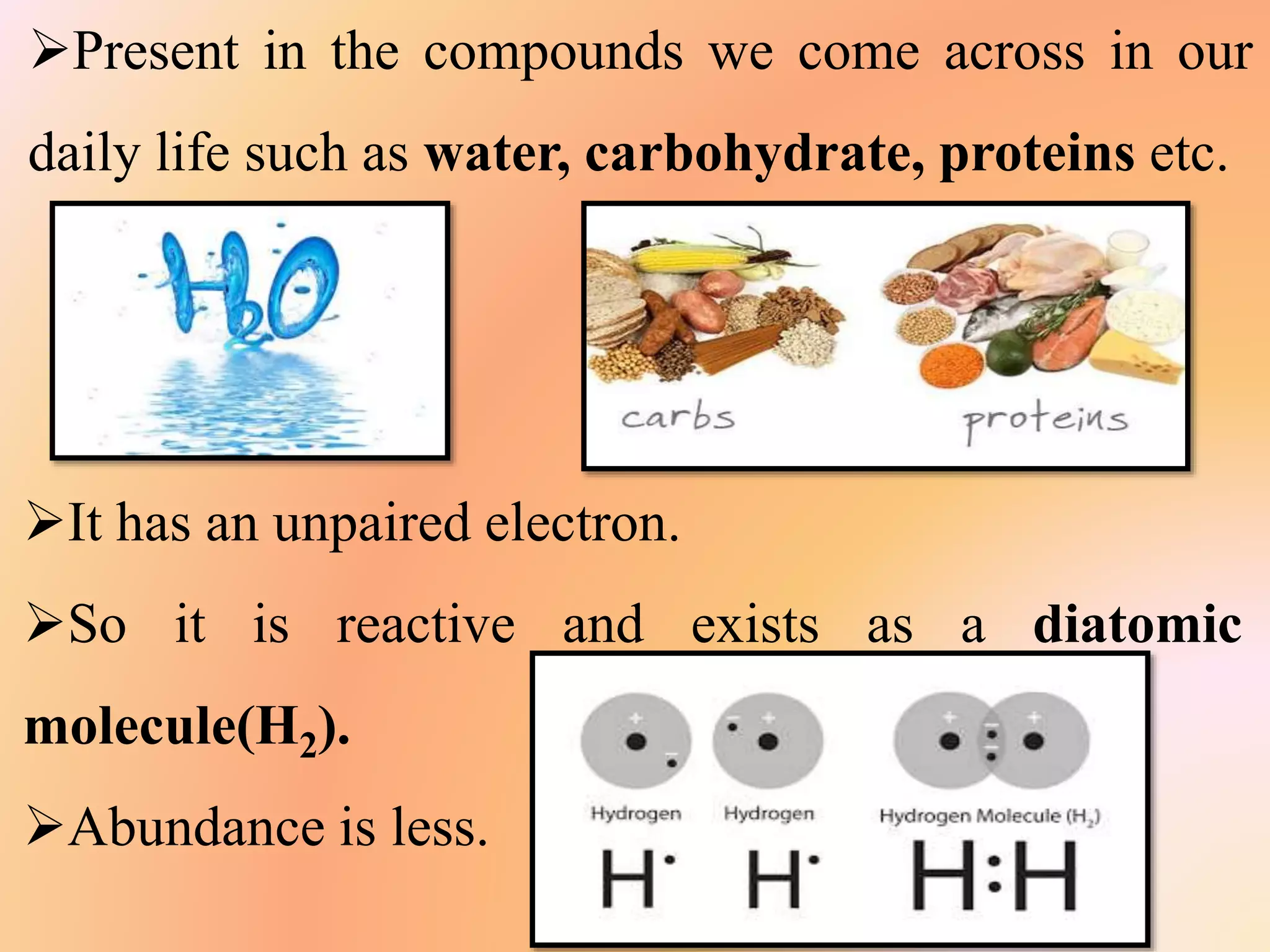



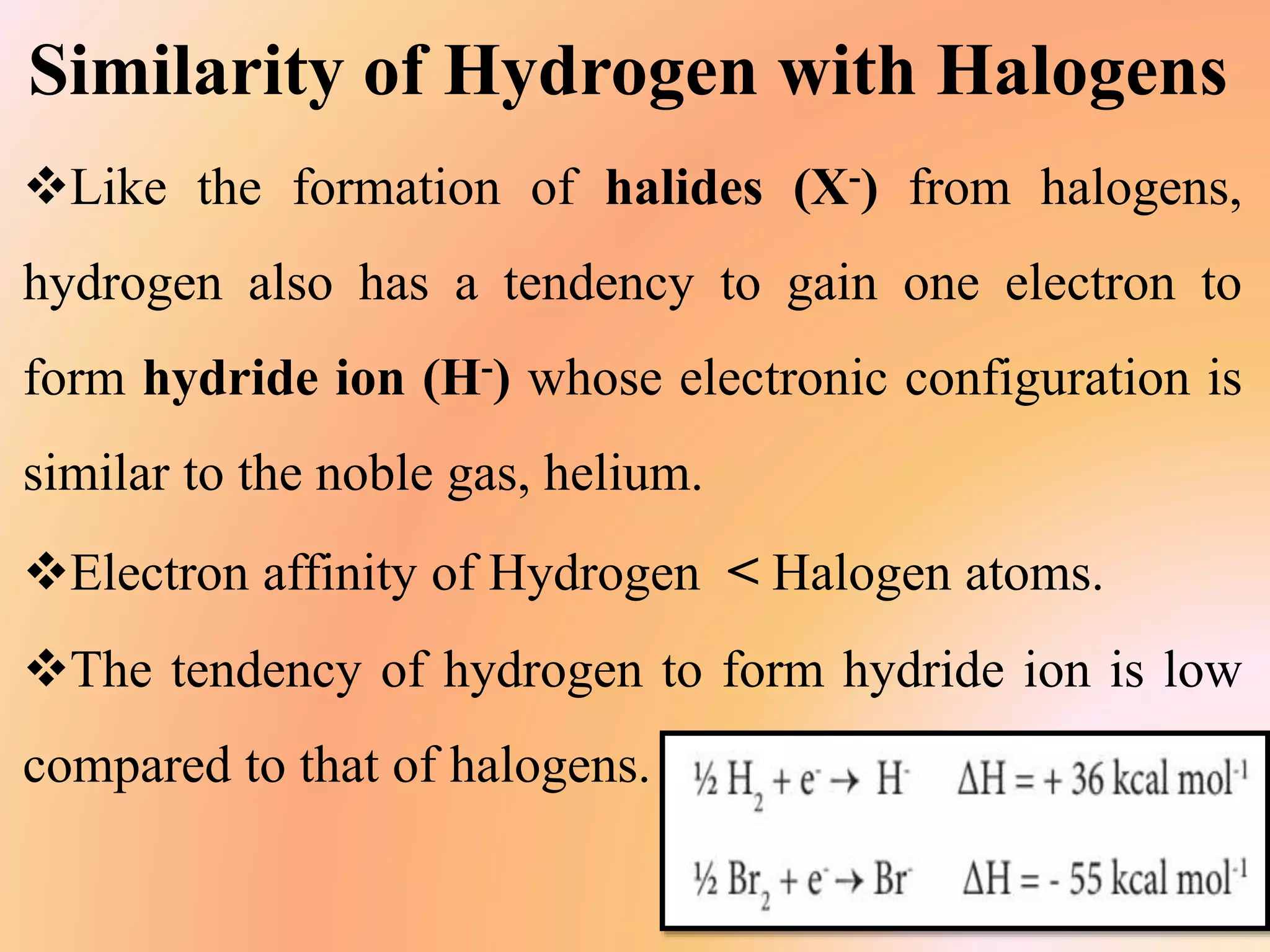

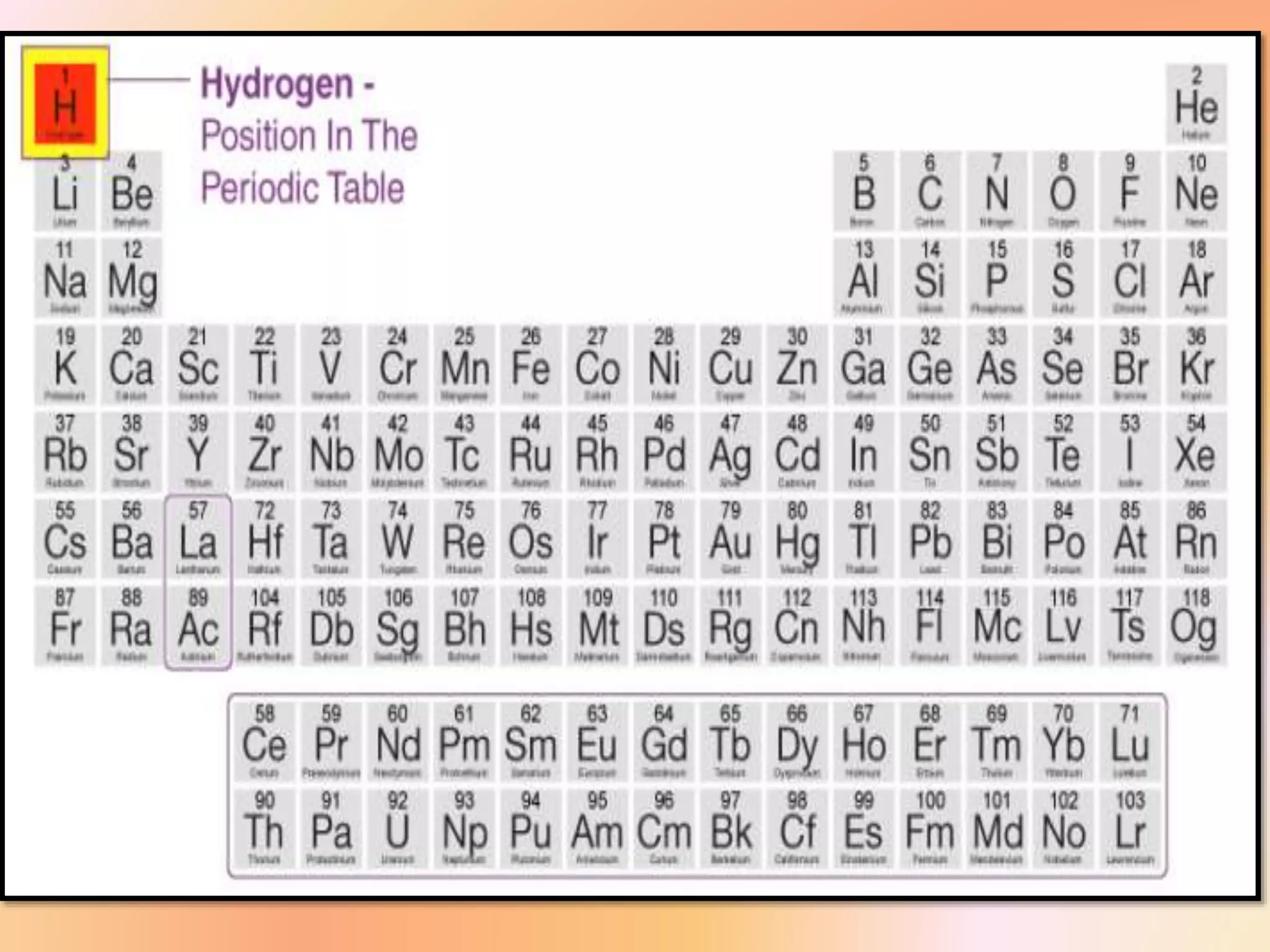







































































The document discusses hydrogen's position in the periodic table, its isotopes (protium, deuterium, tritium), and their properties. It covers hydrogen's preparation, uses, and reactions with various elements, alongside water's characteristics, types of hardness, and methods of hardness removal. Additionally, it highlights heavy water and hydrogen peroxide, detailing their properties and applications.
















































































