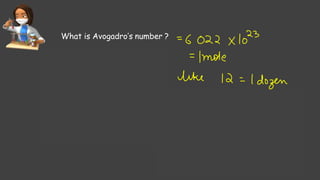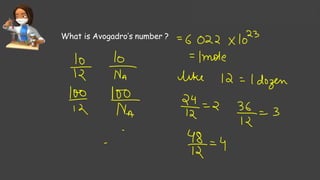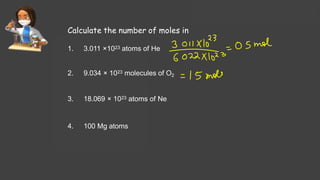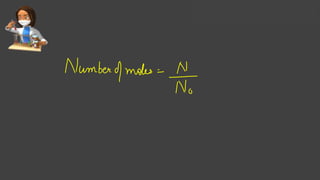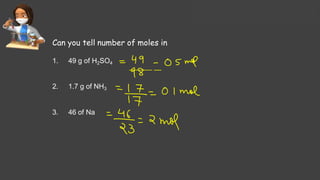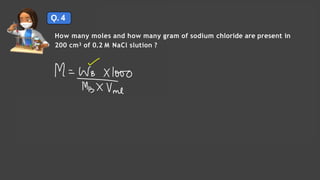This document contains lecture notes on solutions chemistry concepts including:
- Avogadro's number and using it to calculate moles of substances
- Definitions of atomic mass, molecular mass, gram atomic/molecular mass
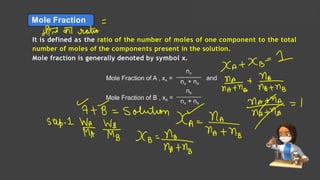
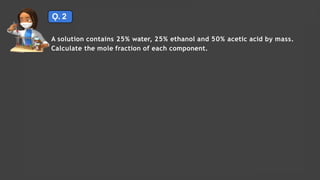
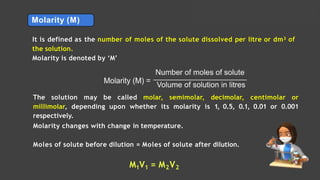
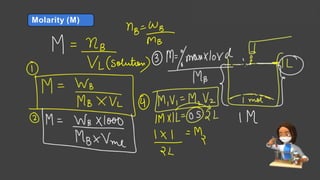
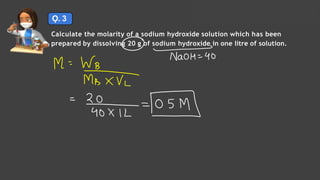
- Expressing concentration of solutions using molarity, mole fraction, molality, and other units
- Sample calculations are shown for converting between grams and moles of substances and determining molarity and mole fraction of solutions