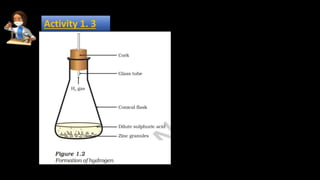
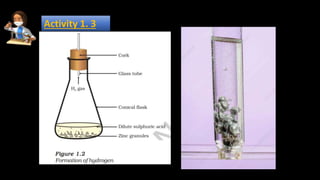
The document provides information about various chemical reactions:
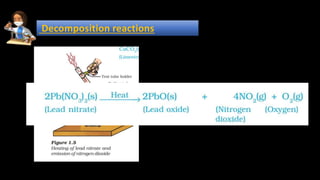
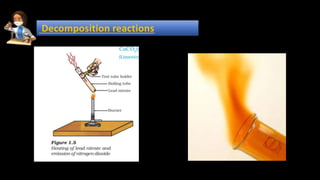
1. Decomposition reactions involve a single reactant breaking down into simpler products, such as ferrous sulfate decomposing into ferric oxide, sulfur dioxide, and sulfur trioxide when heated.
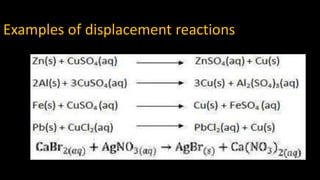
2. Displacement reactions occur when a more reactive element displaces a less reactive element from its compound, like iron displacing copper from copper sulfate solution.
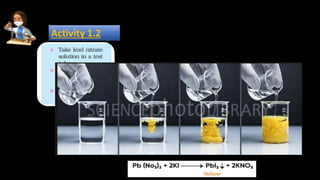
3. Double displacement reactions involve the switching of ions between reactants to form new ionic compounds, exemplified by the reaction of barium chloride and sodium sulfate forming barium sulfate and sodium chloride.
4. Combination reactions form a single product from two or more reactants, such as calcium oxide react