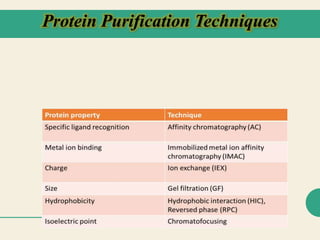
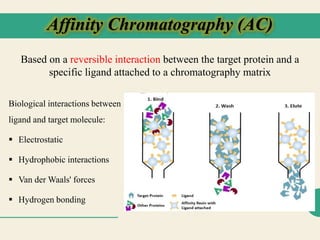
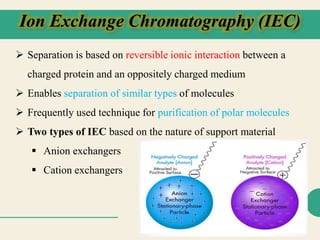
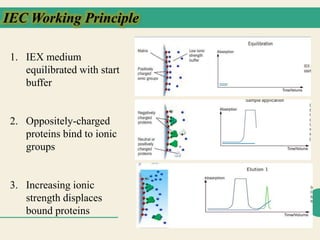
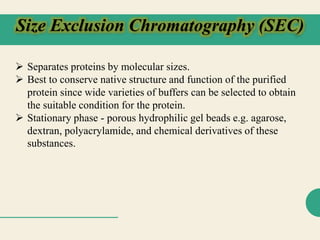
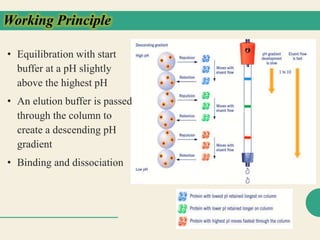
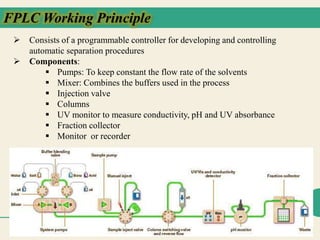
Protein purification techniques allow researchers to isolate a single protein type from complex biological mixtures. Chromatography is commonly used, separating proteins based on differences in their chemical, structural, and functional properties. Affinity chromatography exploits specific biological interactions between a ligand and target protein. Other techniques include ion exchange chromatography, which separates proteins based on charge; size exclusion chromatography, which separates by molecular size; and chromatofocusing, which separates according to isoelectric point. Automated systems now allow for high-throughput protein purification.