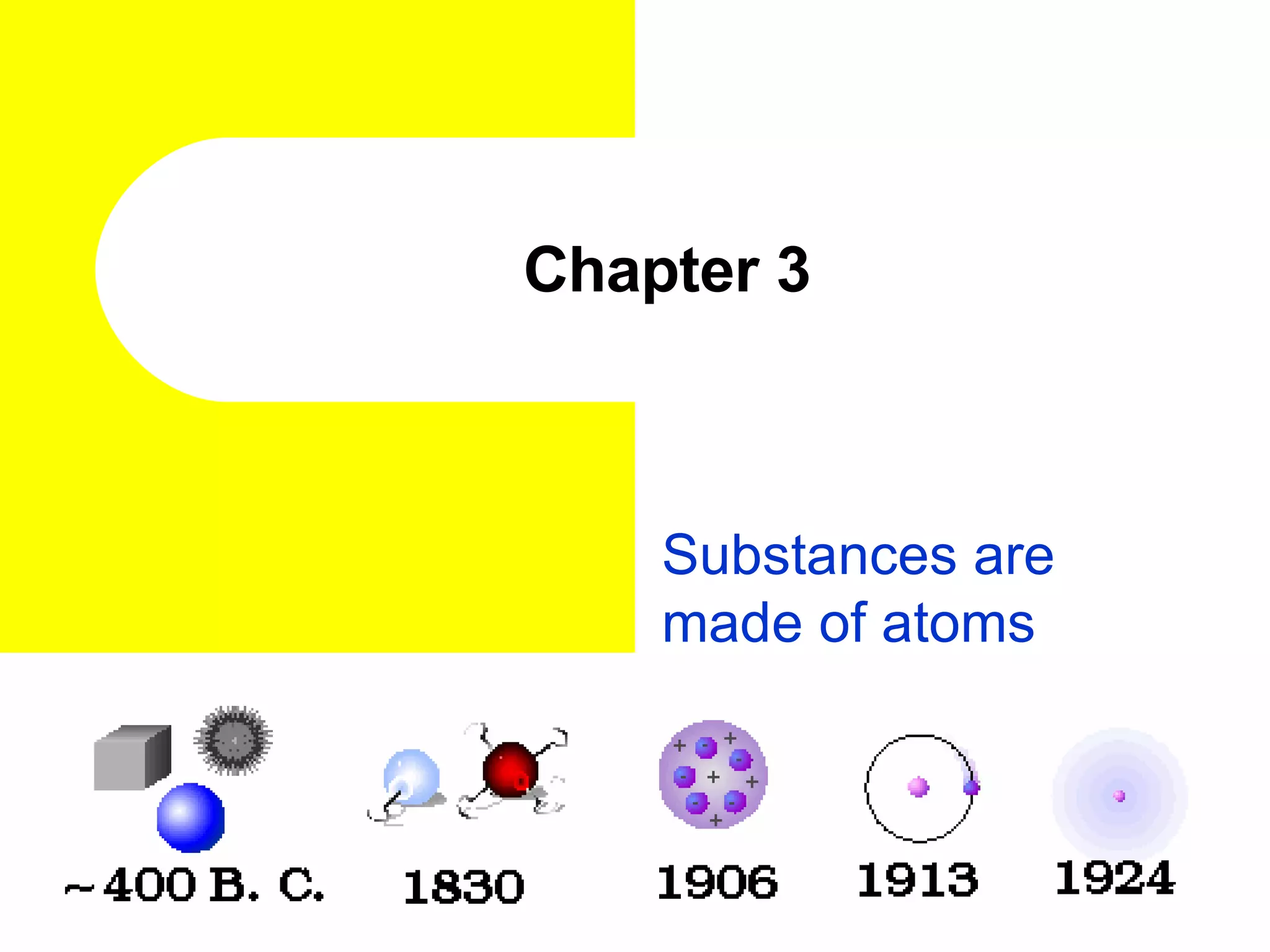
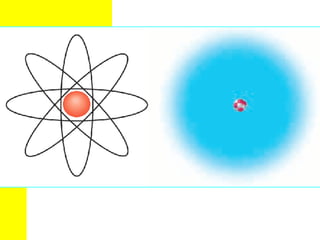
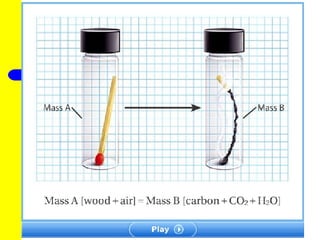
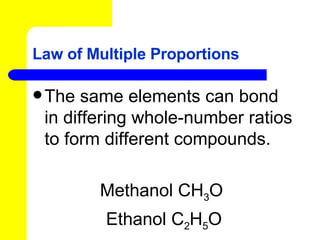
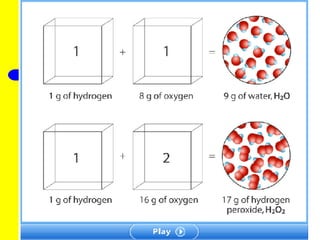
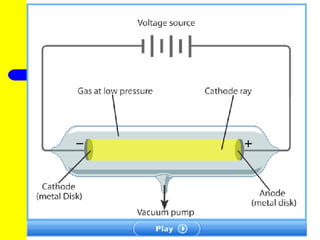
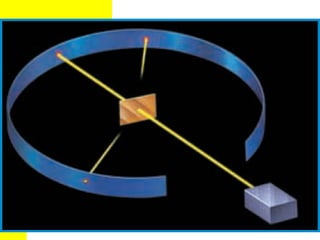
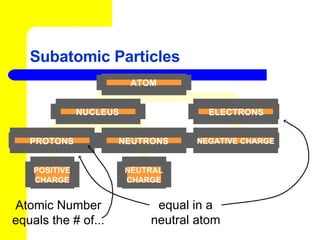
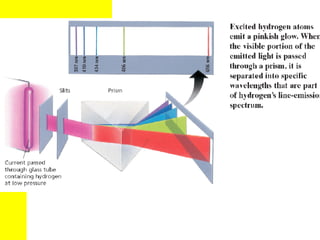
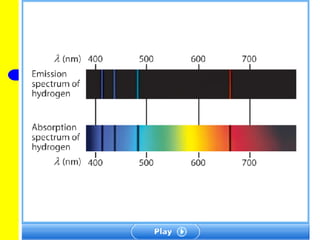
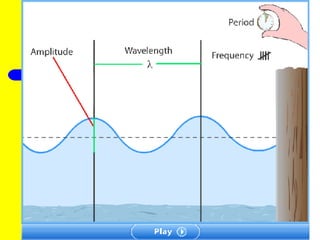
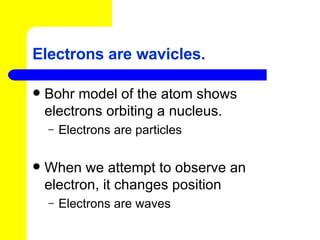
The document discusses the history of atomic theory from ancient Greek philosophers to modern atomic structure. It introduces key figures like Democritus, Dalton, Thomson, and Rutherford and their contributions to understanding that all matter is made of tiny indivisible particles called atoms. The document also describes the discovery of subatomic particles like protons, neutrons, and electrons, and how electrons occupy distinct energy levels around the nucleus in an atom.