Embed presentation
Downloaded 340 times

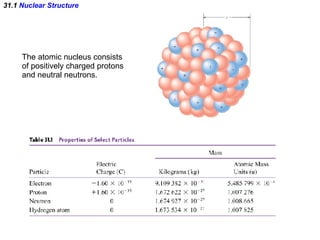
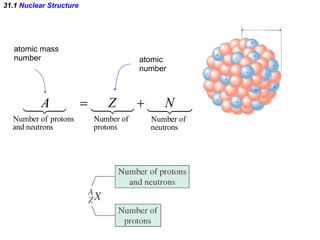
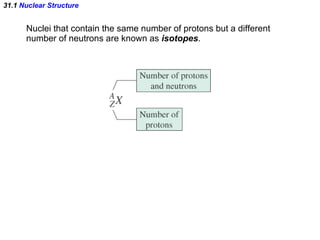
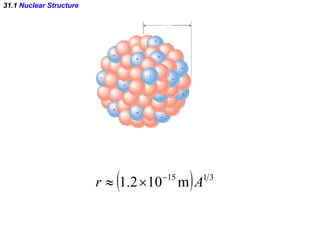
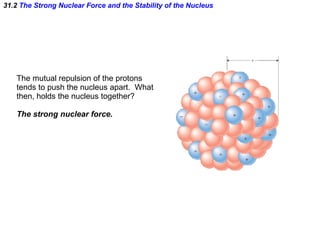
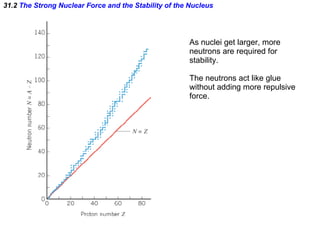
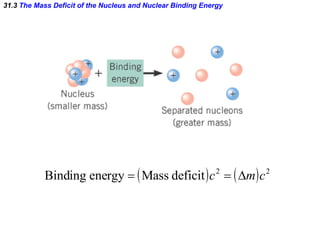
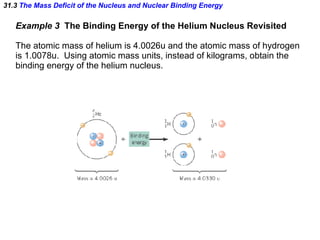
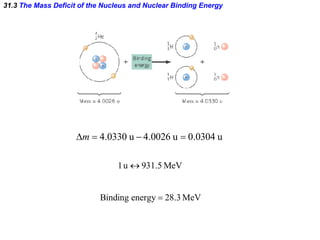
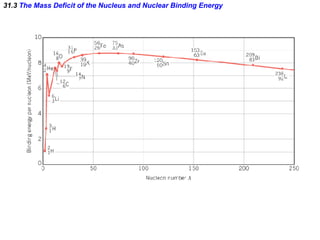
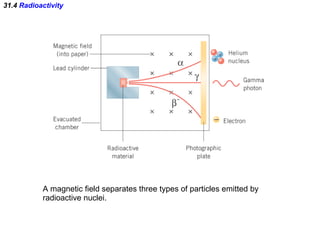
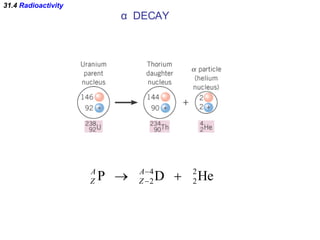
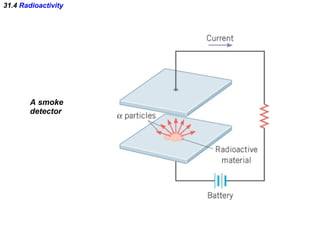
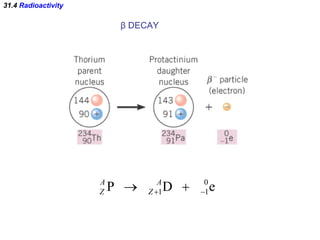
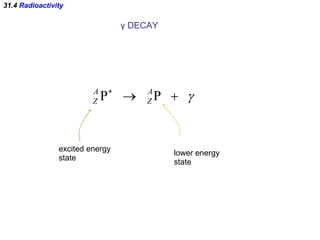
The document discusses nuclear physics and radioactivity. It describes the structure of atomic nuclei consisting of protons and neutrons. Isotopes are nuclei that contain the same number of protons but different neutrons. The strong nuclear force holds the nucleus together by counteracting the repulsive force between protons. As nuclei get larger, more neutrons are required for stability. Nuclear binding energy is released when nuclei fuse because the actual mass is less than the sum of the individual parts. There are three types of particles, alpha, beta, and gamma, emitted during radioactive decay as nuclei move to more stable configurations.















