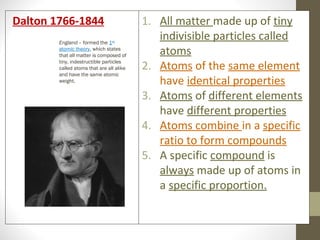
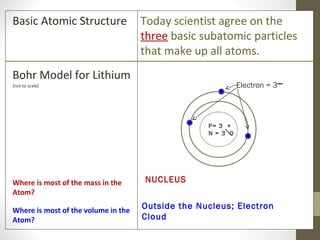
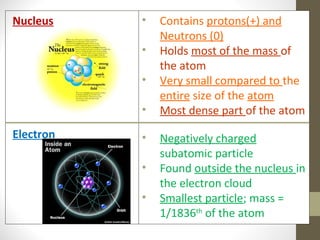
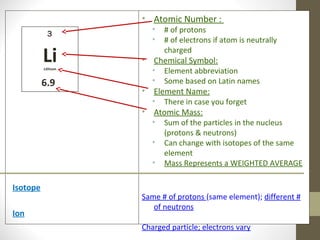
The document summarizes key developments in the atomic theory model over time from ancient Greece to the 20th century. It discusses early ideas from Democritus and Dalton's first atomic theory. Major discoveries include Thomson discovering the electron and proposing the plum pudding model, Rutherford using the gold foil experiment to show the atom's structure with a dense nucleus, and Bohr introducing the planetary atomic model. Later, Schrodinger introduced the wave mechanic model and quantum theory. The basic atomic structure is then explained, identifying the subatomic particles - protons, neutrons, and electrons - and their properties. Key terms like atomic number, isotopes, and ions are also defined.