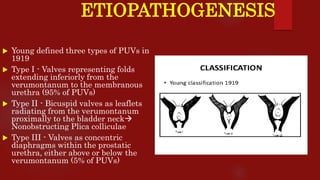
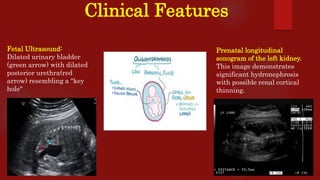
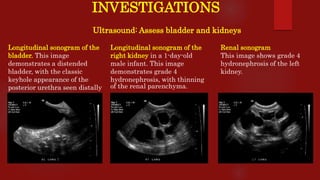
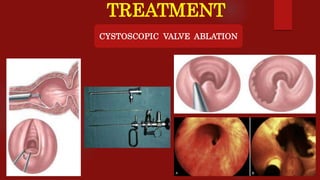
Posterior urethral valves (PUVs) are the most common congenital cause of bladder outlet obstruction in boys, leading to kidney and bladder development issues. The document outlines the pathophysiology, clinical features, diagnosis, treatment options, and long-term follow-up for PUVs, emphasizing the importance of managing renal function and bladder health. Prognosis is often poor, with a significant risk of end-stage renal failure and complications affecting sexual function.