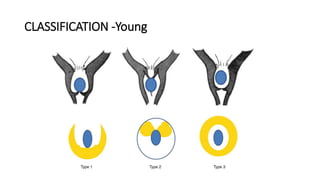
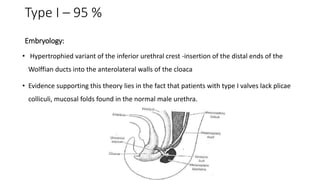
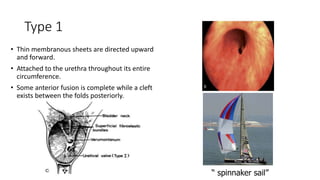
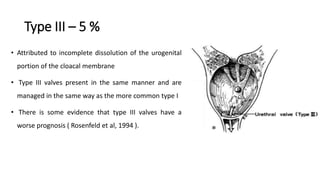
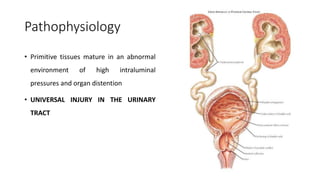
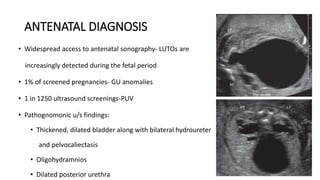
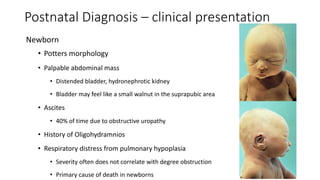
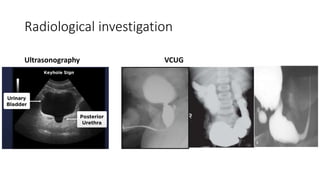
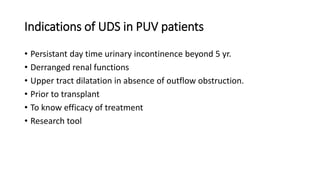
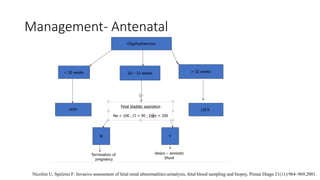
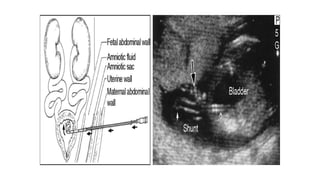
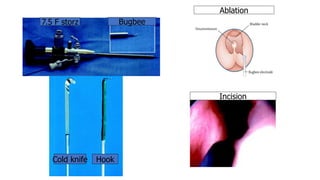
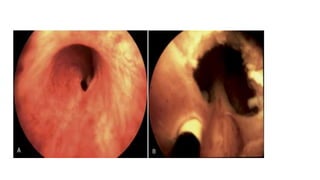
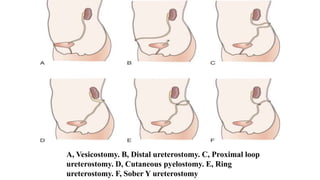
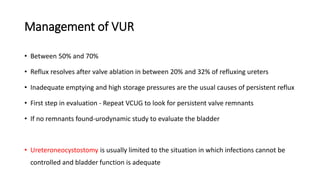
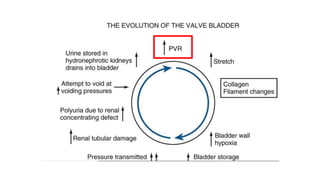
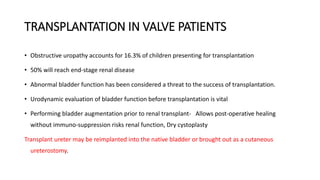
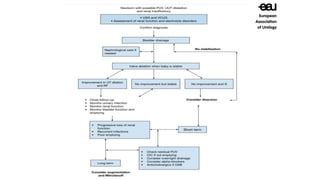
The document discusses posterior urethral valves (PUV), highlighting their history, prevalence, classification, pathophysiology, diagnosis, and management strategies. PUV leads to various complications such as renal dysplasia, bladder dysfunction, and potential kidney failure, emphasizing the need for both antenatal and postnatal diagnosis, with a focus on surgical interventions and ongoing care. Prognostic indicators for renal function are also covered, noting that early diagnosis and management significantly influence long-term outcomes.