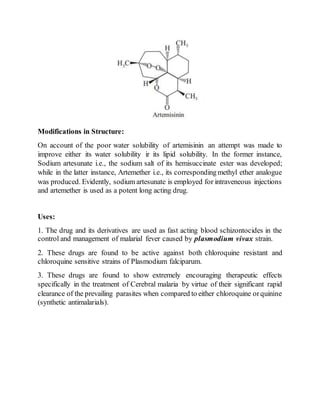
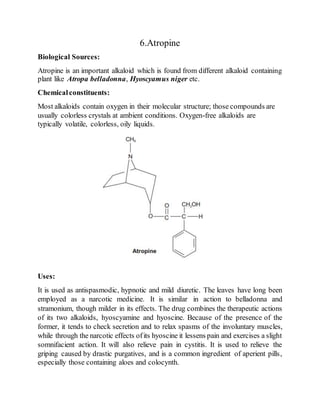
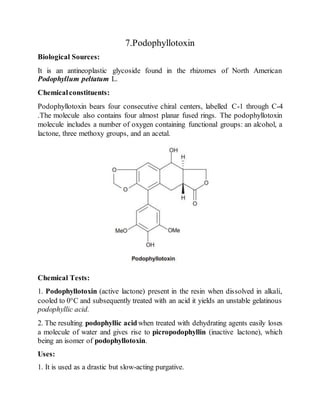
The document is an assignment on phytoconstituents compiled by Al Riyad Hasan from Jashore University of Science and Technology. It details various phytoconstituents such as forskolin, sennosides, artemisinin, diosgenin, digoxin, atropine, podophyllotoxin, caffeine, taxol, vincristine, and vinblastine, along with their biological sources, chemical constituents, and uses. It serves as a comprehensive overview of these compounds and their significance in pharmacology.






![Uses:
1. Taxol is primarily employed in the treatment and management of metastatic
carcinoma of the ovarian glands after the failure of follow-up chemotherapy.
2. It is also used in the treatment of breast cancer usually after the observed failure
of combination chemotherapy for metastatic disease.
3. Because of its hydrophobic nature the injectable concentrate of taxol formulation
meant for intravenous infusion is normally solubilized duly in polyoxyethylated
caster oil. However, before injection it should be appropriately diluted in normal
saline or dextrose solution or combination thereof.
10.Vincristine
Synonyms:
Leurocristine; VCR; LCR.
Biological Sources:
It is also obtained from Vinca rosea Lin., (Catharanthus roseus G. Don)
belonging to the natural order Apocynaceae.
Chemical Characteristic Features:
It may be named as: 22-Oxovincaleukoblastine.
1. It is obtained as blades from methanol having mp 218-220°C.
2. Its specific optical rotation [α]25 D + 17°; [α]25 D + 26.2° (ethylene chloride);
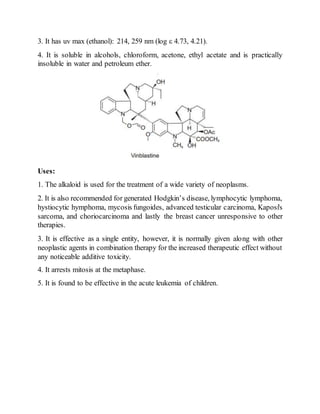
pKa: 5.0, 7.4 in 33% DMF.](https://image.slidesharecdn.com/cover-copy-190418040743/85/Phytoconstituents-13-320.jpg)
![3. It has uv max (ethanol): 220, 255, 296 nm (log am 4.65, 4.21, 4.18).
Identification Tests:
Vincristine Sulphate (C46H56N4O10.H2SO4) (Vincrex, Oncovin, Vincosid,
Kyocrystine): Its crystals are obtained from ethanol and is found to be unstable.
Uses:
1. Vincristine sulphate is recommended for the treatment of acute lymphocytic
leukemia, and in combination therapy in Hodgkin's disease, lymphosarcoma,
reticulum cell sarcoma, neuroblastoma, Wilm's tumour and rhabdomyosarcoma.
Viucristine sulphate being highly unstable; therefore, its refregerated storage in
sealed ampules is absolutely essential.
2. It is broadly used as an antineoplastic agent.
11.Vinblastine
Synonyms:
Vincaleukoblastine; VLB; 29060-LE;
Biological Source:
It is obtained from Vinca rosea Lin.. (Apocynaceae).
Chemical Characteristic Features:
1. It is obtained as solvated needles from methanol having mp 211-216°C.
2. Its specific optical rotation [α]26 D + 42° (chloroform).](https://image.slidesharecdn.com/cover-copy-190418040743/85/Phytoconstituents-14-320.jpg)