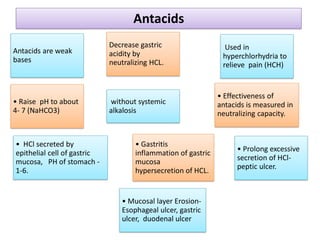
This document discusses various gastrointestinal agents used to treat gastrointestinal disorders, including acidifiers, antacids, adsorbents, and cathartics. It provides details on specific agents such as ammonium chloride, dilute hydrochloric acid, and aluminum hydroxide gel. It explains that antacids are weak bases that neutralize stomach acid and relieve pain by raising the gastric pH to between 4-7 without causing systemic alkalosis. The document also outlines the ideal characteristics of antacids and categorizes them as either systemic/absorbable or non-systemic.