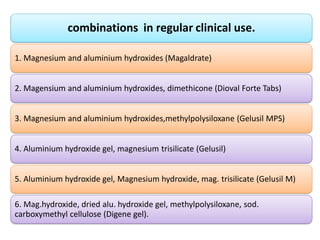
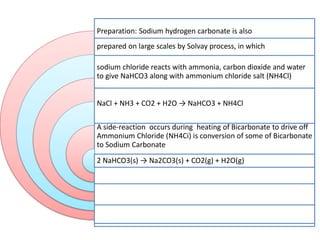
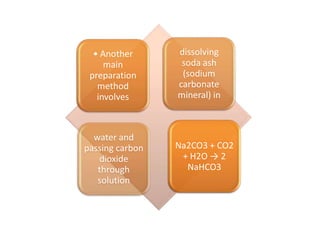
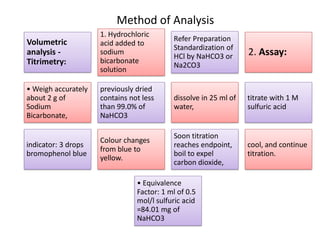
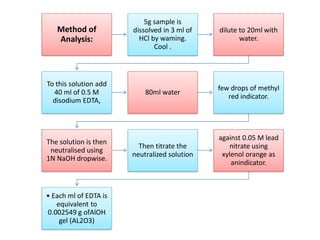
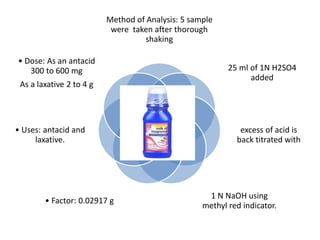
This document discusses various antacids, including their compositions, mechanisms of action, preparations, and analysis methods. It covers common antacid combinations containing aluminum hydroxide gel, magnesium hydroxide, magnesium trisilicate, and sodium bicarbonate. Individual antacids like milk of magnesia and aluminum hydroxide gel are also described, outlining their chemical properties, uses as antacids, and methods for quantitative analysis. Preparation processes are provided for sodium bicarbonate and analysis methods involve titrimetric techniques.