Carbohydrate metabolism b.pharm
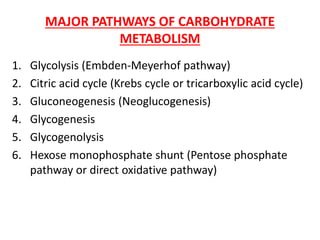
- 1. MAJOR PATHWAYS OF CARBOHYDRATE METABOLISM 1. Glycolysis (Embden-Meyerhof pathway) 2. Citric acid cycle (Krebs cycle or tricarboxylic acid cycle) 3. Gluconeogenesis (Neoglucogenesis) 4. Glycogenesis 5. Glycogenolysis 6. Hexose monophosphate shunt (Pentose phosphate pathway or direct oxidative pathway)
- 3. GLYCOLYSIS (EMBDEN-MEYERHOF PATHWAY) -Significance of glycolysis pathway- • Only pathway taking place in all the cells of the body (universal pathway). • Only source of energy in tissues lacking mitochondria, e.g. erythrocytes, cornea, lens etc. • In absence of enough O2, anaerobic glycolysis forms the major source of energy for muscles. • The preliminary step before complete oxidation. • Provides carbon skeletons for synthesis of non-essential amino acids as well as glycerol part of fat. • Reversible reactions of glycolysis are used for gluconeogenesis. • Brain is dependent on glucose for energy. The glucose in brain has to undergo glycolysis before it is oxidized to CO2 and H2O.
- 4. -Introduction- • Glycolysis is derived from the Greek words (glykys = sweet; and lysis = dissolution or splitting). • The complete pathway of glycolysis was elucidated in 1940. • Glycolysis was the first metabolic pathway to be elucidated and is probably the best understood. -Definition- • Glycolysis is defined as the sequence of reactions converting glucose (or glycogen) to two molecules of three-carbon compound pyruvate (aerobic condition) or lactate (anaerobic condition), with the production of ATP. -Site of reactions- • All the reaction steps take place in the cytoplasm.
- 5. -Entry of glucose into cells- • Glucose transporter-4 (GluT4) transports glucose from the extracellular fluid to muscle cells and adipocytes under the influence of insulin. • Insulin promotes the translocation of intracellular GluT4 molecules to the cell surface and thus increases glucose uptake. • In Type 2 diabetes mellitus, membrane GluT4 is reduced, leading to insulin resistance in muscle and fat cells. • In diabetes, entry of glucose into muscle is only half of normal cells. • Glucose transporter-2 (GluT2) transports glucose in the liver cells without the influence of insulin.
- 6. Glucose transporters Transporter Present in Properties GluT1 RBC, brain, kidney, colon, retina, placenta Glucose uptake in most of cells GluT2 Serosal surface of intestinal cells, liver, beta cells of pancreas Low affinity; glucose uptake in liver; glucose sensor in beta cells GluT3 Neurons, brain High affinity; glucose into brain cells GluT4 Skeletal, heart muscle, adipose tissue Insulin-mediated glucose uptake GluT5 Small intestine, testis, sperms, kidney Fructose transporter; poor ability to transport glucose GluT7 Liver endoplasmic reticulum Glucose from ER to cytoplasm SGluT Intestine, Kidney Cotransport; from lumen into cell
- 8. Comparison of hexokinase and glucokinase Hexokinase Glucokinase Occurence In all tissues Only in liver Km value 10-2 mmol/L 20 mmol/L Affinity to substrate High Low Specificity Acts on glucose, fructose and mannose Acts only on glucose Induction Not induced Induced by insulin and glucose Function Even when blood sugar level is low, glucose is utilized by body cells Acts only when blood glucose level is more than 100 mg/dL; then glucose is taken up by liver cells for glycogen synthesis.
- 9. The over all equation for glycolysis under aerobic conditions : Glucose + 2ATP + 2NAD+ + 4ADP + 2Pi 2 pyruvate + 2 ADP + 2NADH + 2H+ + 4ATP + 2H2O
- 10. -Regulation of Glycolysis- • The glycolytic pathway has dual role. i) It degrades glucose to generate ATP. ii) It provides building blocks (precursors) for synthetic reactions such as the formation of fatty acids. • The rate of conversion of glucose into pyruvate is regulated to meet these two major cellular needs. • In metabolic pathways, enzymes catalyzing irreversible reactions are potential sites of control. • In glycolysis, the reactions catalyzed by : hexokinase, phosphofructokinase-I (PFK) and pyruvate kinase are virtually irreversible and function as regulatory enzymes. The activity of these enzymes is regulated either by: i) Allosteric effectors ii) Covalent modification iii) By increasing (induction) or decreasing (repression) the amounts of these enzymes at the level of transcription.
- 11. Regulation of glycolysis in muscle • Glycolysis in muscle provides ATP for contraction. Control of glycolysis in muscle depends on the ratio of ATP to AMP. 1. Hexokinase • Hexokinase is an allosteric enzyme catalyzing the first step of glycolysis is inhibited by its product glucose-6-phosphate in extra-hepatic tissues. • High concentration of glucose-6-phosphate signal that the cell no longer requires glucose for energy or for the synthesis of glycogen and the glucose will be left in the blood. • When phosphofrucktokinase-I is inactive, the concentration of fructo-6-phosphate rises. • In turn, the level of glucose-6-phosphate rises. • Hence, the inhibition of phosphofrucktokinase-I lead to the inhibition of hexokinase. 2. Phosphofructokinase-I • High levels of ATP allosterically inhibit the enzyme by lowering the phosphofructokinase-I (PFK) enzyme’s affinity for fructose-6-phosphate its substrate. • AMP reverses the inhibitory action of ATP and so the activity of the enzyme increases when the ATP/AMP ratio is lowered. • A decrease in pH also inhibits phosphofructokinase-I activity by enhancing the inhibitory effect of ATP.
- 12. 3. Pyruvate kinase • Pyruvate kinase is the enzyme catalyzing the third irreversible step in glycolysis. • This step yields ATP and pyruvate that can be oxidized further or used as a precursor for synthetic reactions. • ATP allosterically inhibits pyruvate kinase to slow glycolysis when energy level is high. • Alanine synthesized from pyruvate in muscle also allosterically inhibits pyruvate kinase but in this case, it signals that precursors are abundant.
- 13. Regulation of glycolysis in liver 1. Phosphopfructokinase • Regulation with respect to ATP is the same in the liver as in muscle. • Low pH is not a metabolic signal for the liver enzyme, because lactate is not normally produced in the liver. • In liver phosphofructokinase is inhibited by citrate, an intermediate in the citric acid cycle. • A high levels of citrate in the cytoplasm indicates that biosynthetic precursors are abundant, and so there is no need to degrade additional glucose for this purpose. • Citrate inhibits phosphofructokinase by enhancing the inhibitory effect of ATP. • One more means by which glycolysis in the liver is regulated is through fructose-2,6-bisphosphate; a potent activator of phosphrofructokinase-I. • In the liver, the concentration of fructose-6-phosphate rises when blood glucose concentration is high, and the abundance of fructose-6-phosphate accelerate the synthesis of F-2,6-BP. • The binding of F-2,6-BP increases the affinity of phosphofructokinase-I for fructose-6-phosphate and diminishes the inhibitory effect of ATP.
- 14. 2. Hexokinase (Glucokinase) • Regulation of hexokinase reaction is the same in the liver as in muscle. However, liver possesses another specialized isoenzyme of hexokinase, called glucokinase, which is not inhibited by glucose-6-phosphate. • Glucokinase phosphorylates glucose only when glucose is abundant because the affinity of glucokinase for glucose is about 50-fold lower than that of hexokinase. • The role of glucokinase is to provide glucose-6-phosphate for the synthesis of glycogen and for the formation of fatty acids. • The activity of glucokinase is thus influenced by carbohydrate intake. The activity increases with carbohydrate intake and decreases during starvation and diabetes mellitus. • Liver glucokinase is inducible enzyme that increases its synthesis in response to insulin and decreases in response to glucagon, • Insulin signals the need to remove glucose from the blood for storage as glycogen or conversion into fat.
- 15. 3. Pyruvate kinase • Several isoenzymic forms of pyruvate kinase are present. • The L type predominates in the liver, and the M type in muscle and the brain. • The catalytic properties of the liver pyruvate kinase (L form) but not the muscle pyruvate kinase (M form) are controlled by reversible phosphorylation. • When the blood glucose level is low, the glucagon-triggered cAMP leads to the phosphorylation of pyruvate kinase, which reduces its activity. • The hormone-triggered phosphorylation prevents the liver from consuming glucose when it is needed by the brain an muscle.
- 16. Possible fates of pyruvate formed in glycolysis
- 17. • Pyruvate is reduced to lactate in presence of the enzyme lactate dehydrogenase. Significance of lactate production • For smooth operation of the pathways, the NADH is to be reconverted to NAD+ . This can be done by oxidative phosphorylation. However, during exercise, there is lack of oxygen. So, this reconversion is not possible. Hence, pyruvate is reduced to lactate; the NAD+ thus generated is reutilized for uninterrupted operation of the 5th step of glycolysis. • In RBCs, there are no mitochondria. Hence, RBCs derive energy only through glycolysis, where the end product is lactic acid. • Brain, retina, skin, renal medulla and gastrointestinal tract derive most of their energy from glycolysis. Fate of Pyruvate under anaerobic condition (lack of O2)
- 18. -Significance of lactate production- Fig. Lactate formation is necessary for reconversion of NADH to NAD+ during anaerobiasis
- 19. CORI’S CYCLE OR LACTIC ACID CYCLE • It is a process in which glucose is converted to lactate in the muscle and in the liver this lactate is reconverted into glucose. • In an actively contracting muscle, pyruvate is reduced to lactic acid which may tend to accumulate in the muscle. The muscle cramps, often associated with strenous muscular exercise are thought to be due to lactate accumulation. • To prevent the lactate accumulation, body utilizes Cori’s cycle. • Lactic acid from muscle diffuses into the blood. • Lactate then reaches liver where it is oxidized to pyruvate. Thus, it is channelled to gluconeogenesis. • Regenerated glucose can enter into blood and then to muscle. • This cycle is called Cori’s cycle.
- 20. Significance of Cori’s cycle • The lactate produced in the muscle is efficiently reutilized by the body. But this is an energy – expensive process. • During exercise, lactate production is high, which is utilized by liver to produce glucose. The process needs ATP. • The resultant increased oxygen consumption is the explanation for the oxygen debt after vigrous exercise. Fig. Cori's cycle. Contracting muscle has lack of oxygen. So pyruvate is reduced to lactate. This can be reconverted to glucose in liver by gluconeogenesis
- 21. Glycolysis and fermentation -Introduction- • The earliest cells lived in an atmosphere almost devoid of oxygen and had to develop strategies for deriving energy from fuel molecules under anaerobic conditions. • Most modern organisms have retained the ability to constantly regenerate NAD+ during anaerobic glycolysis by transferring electrons from NADH to form a reduced end product such as lactate or ethanol. • Some tissues and cell types (such as erythrocytes, which have no mitochondria and thus cannot oxidize pyruvate to CO2) produce lactate form glucose even under aerobic conditions. • Fermentation is the general term for such processes, which extract energy (as ATP) but do not consume oxygen or change the concentrations of NAD+ or NADH. • Fermentations are carried out by a wide range of organisms, many of which occupy anaerobic niches, and they yield a variety of end products, some of which find commercial uses.
- 22. -Reactions- • Yeast and other microorganisms ferment glucose to ethanol and CO2, rather than to lactate. • Glucose is converted to pyruvate by glycolysis, and the pyruvate is converted to ethanol and CO2 in a two-step process. • In the first step, pyruvate is decarboxylated in an irreversible reaction catalyzed by pyruvate decarboxylase. • Pyruvate decarboxylation requires Mg2+ and has a tightly bound coenzyme, thiamine pyrophosphate. • In the second step, acetaldehyde is reduced to ethanol through the action of alcohol dehydrogenase, with the reducing power furnished by NADH derived from the dehydrogenation of glyceraldehyde 3-phosphate.
- 23. • Ethanol and CO2 are thus the end products of ethanol fermentation, and the overall equation is Glucose + 2ADP + 2Pi 2 ethanol + 2CO2 + 2ATP + 2H2O • Pyruvate decarboxylase is present in brewer’s and baker’s yeast and in all other organisms that ferment glucose to ethanol, including some plants. • The CO2 produced by pyruvate decarboxylase in brewer’s yeast is responsible for the characteristic carbonation of champagne. • In baking, CO2 released by pyruvate decarboxylase when yeast is mixed with a fermentable sugar causes dough to rise. • The enzyme is absent in vertebrate tissues and in other organisms that carry out lactic acid fermentation. • Alcohol dehydrogenase is present in many organisms that metabolize ethanol. Including humans. -Use of fermentation- • Yogurt, already known in Biblical times, is produced when bacterium Lactobacillus bulgaricus ferments the carbohydrate in milk, producing lactic acid; the resulting drop in pH causes the milk proteins to precipitate, producing the thick texture and sour taste of unsweetened yogurt.
- 24. • Bacterium propionibacterium freudenreichii, ferments milk to produce propionic acid and CO2; the propionic acid precipitates milk proteins, and bubbles of CO2 cause the holes characteristic of Swiss cheese. • Many other food products such as pickles, sauerkraut, sausage, soy sauce, and a variety of national favorities, such as kimchi (Korea), tempoyak (Indonesia), kefir (RussiaI), dahi (Nepal), and pozol (Mexico). • The drop in pH associated with fermentation also helps to preserve foods, because most of the microorganisms that cause food spoilage cannot grow at low pH. • In agriculture, plant biproducts such as corn stalks are preserved for use as animal feed by packing them into a large container (a silo) with limited access to air; microbial fermentation produces acids that lower the pH. The silage that results from this fermentation process can be kept as animal feed for long periods without spoilage.
- 25. -Dental caries- • Common condition especially in children who are fond of taking sucrose-rich food items such as candies, chocolates, ice creams etc. and do not take proper care of the oral cavity, such as mouth rinsing and brushing of teeth after meals. • Characterized by destruction of the tooth enamel as a result of the action of the microorganisms (normally present in the oral cavity e.g. Streptococcus mutans, Lactobacillus sps). • Bacteria residing normally in the oral cavity ferment sucrose into lactic acid and other organic acids which slowly dissolve the enamel. • The local environment becomes favourable for pathogenic bacteria as well. • If undetected, there is gross damage of the teeth and gums commonly known as dental decay and gingivitis. • Low levels of fluoride, from tooth pastes or when applied topically can inhibit the enzyme enolase and reduce glycolysis and thus tooth decay. • Further, fluoride integrates into tooth surface to form fluoroapatite which offers resistance to demineralization.
- 27. Cancer and glycolysis • Glucose uptake and glycolysis proceed about ten times faster in most solid tumors than in non-cancerous tissues. • Tumor cells commonly experience hypoxia (limited oxygen supply), because they initially lack an extensive capillary network to supply the tumor with oxygen. As a result, cancer cells more than 100 to 200 μm from the nearest capillaries depend on anaerobic glycolysis for much of their ATP production. • The high glycolytic rate may also result in part from the smaller numbers of mitochondria in tumor cells; less ATP made by respiration-linked phosphorylation in mitochondria means more ATP is needed from glycolysis. • In addition, some tumor cells overproduce several glycolytic enzymes, including isoenzyme of hexokinase that associates with the cytosolic face of the mitochondrial inner membrane and is insensitive to feedback inhibition by glucose 6-phosphate. • This enzyme may monopolize the ATP produced in mitochondria, using it to convert glucose to glucose 6-phosphate and committing the cell to continued glycolysis.
- 28. • The hypoxia-inducible transcription factor (HIF-1) is a protein that acts at the level of mRNA synthesis to stimulate the synthesis of at least eight of the glycolytic enzymes. This gives the tumor cell the capacity to survive anaerobic conditions until the supply of blood vessels has caught up with tumor growth. • One of the modalities of cancer treatment is to use drugs that can inhibit vascularization of tumors. • Although cancer cells exhibit increased glycolysis and depend more on this pathway for ATP generation, inhibition of glycolysis alone may not be sufficient to effectively kill the malignant cells. It has been suggested that ATP depletion should reach certain thresholds in order to trigger cell death by apoptosis or necrosis processes, with a depletion of 25–70% ATP leading to apoptosis, and an over 85% ATP depletion causing necrosis. • Since all cancer cells contain mitochondria, some degree of ATP generation through oxidative phosphorylation is still possible when glycolysis is inhibited. This may compromise the efficiency of glycolytic inhibitors to deplete cellular ATP. • One way to achieve a high level of ATP depletion and improve therapeutic activity is to combine multiple ATP-depleting agents with different mechanisms of action .
- 29. • Indeed, early studies showed that the combination of N- (phosphonacetyl)-L-aspartate (PALA), 6- methylmercaptopurine riboside (MMPR), and 6- aminonicotinamide (6-AN) is an effective ATP-depleting regimen that increases the anticancer activity of radiation, adriamycin, or taxol. • Combination of glycolytic inhibitor 2-deoxyglucose with adriamycin or paclitaxel also resulted in a significant increase of in vivo therapeutic activity in animal tumor models bearing osteosarcoma or non-small- cell lung cancer xenografts.
- 30. Rapaport-Leubering cycle • This is a shunt pathway to glycolysis which is operative in the erythrocytes of man and other mammals. • Rapaport-Leubering cycle is mainly concerned with the synthesis of 2,3-bisphosphoglycerate (2,3-BPG) in the RBC. • 1,3-Bisphosphoglycerate (1,3-BPG) produced in glycolysis is converted to 2,3-BPG by the enzyme 2,3- bisphosphoglycerate mutase. • 2,3-BPG is hydrolyzed to 3- phosphoglycerate by bisphosphoglycerate phosphatase. • About 15-20% of the glucose that gets converted to lactate in erythrocytes goes via 2,3-BPG synthesis.
- 31. Significance of 2,3-BPG • Production of 2,3-BPG allows the glycolysis to proceed without the synthesis of ATP. Rapaport-Leubering cycle, therefore is a shunt pathway of glycolysis to dissipate or waste the energy not needed by the erythrocytes. • 2,3-BPG, is not a waste molecule in RBC. It combines with hemoglobin (Hb) and reduces Hb affinity with oxygen. Therefore, in the presence of 2,3-BPG, oxyhemoglobin unloads more oxygen to the tissues. Increase in erythrocyte 2,3- BPG is observed in hypoxic condition, high altitude, fetal tissues, anemic conditions etc. • Glycolysis in the erythrocytes is linked with 2,3-BPG production and oxygen transport. In the deficiency of the enzyme hexokinase, glucose is not phosphorylated, hence the synthesis and concentration of 2,3-BPG are low in RBC. The hemoglobin exhibits high oxygen affinity in hexokinase-defective patients. On the other hand, in the patients with pyruvate kinase deficiency, the level of 2,3-BPG in erythrocytes is high, resulting in low oxygen affinity. • In hypoxia : the concentration of 2,3-BPG in erythrocytes is elevated in chronic hypoxic conditions associated with difficulty in O2 supply. These include adaptation to high altitude, obstructive pulmonary emphysema (airflow in the bronchioles blocked) etc.
- 32. • In anemia : 2,3-BPG levels are increased in severe anemia in order to cope up with the oxygen demands of the body. This is an adaptation to supply as much as O2 as possible to the tissue, despite the low hemoglobin levels. • In blood transfusion : storage of blood in acid citrate-dextrose medium results in the decreased concentration of 2,3-BPG. Such blood when transfused fails to supply O2 to the tissues immediately. Addition of inosine (hypoxanthine-ribose) to the stored blood prevents the decrease of 2,3-BPG. The ribose moiety of inosine gets phosphorylated and enters the hexose monophosphate pathway and finally gets converted to 2,3-BPG. • Fetal hemoglobin (HbF): The binding of 2,3-BPG ot fetal hemoglobin is very weak. Therefore, FbF has higher affinity for O2 compared to adult hemoglobin (HbA). This may be needed for the transfer of oxygen from the maternal blood to the fetus.
- 34. GLUCONEOGENESIS INTRODUCTION • The brain alone requires about 120 g of glucose each day – more than half of all the glucose stored as glycogen in muscle and liver. However, the supply of glucose from these stores is not always sufficient; between meals and during longer fasts, or after vigorous exercise, glycogen is depleted. • For these times, organisms need a method for synthesizing glucose from non- carbohydrate precursors. This is accomplished by a pathway called gluconeogenesis (“ formation of new sugar”), which converts pyruvate and related three- and four carbon compounds to glucose. • Gluconeogenesis occurs in all animals, plants, fungi, and microorganisms. • The reactions are essentially the same in all tissues and all species. • Although the reactions of gluconeogenesis are the same in all organisms, the metabolic context and the regulation of the pathway differ from one species to another and from tissue to tissue. • The important precursors of glucose in animals are three-carbon compounds such as lactate, pyruvate, and glycerol, as well as certain amino acids.
- 35. DEFINITION • Gluconeogenesis (neoglucogenesis) is the process of formation of glucose or glycogen from various non-carbohydrate precursors such as glucogenic amino acids, lactate, glycerol and propionate. TISSUES ACTIVE IN GLUCONEOGENESIS • Major site: Liver. • Minor site: Kidney. • Very little: – Brain. – Muscle (skeletal and heart). • In liver and kidney it helps to maintain the glucose level in the blood so that brain and muscle can extract sufficient glucose from it to meet their metabolic demands. • About 1 kg glucose is synthesized everyday. SITE • Reactions of gluconeogenesis occur both in mitochondria and cytosol. SIGNIFICANCE OF GLUCONEOGENESIS • Brain and central nervous system, erythrocytes, testes and kidney medulla are dependent on glucose for continuous supply of energy. • Glucose is the only source that supplies energy to the skeletal muscle, under anaerobic conditions. • In fasting even more than a day, gluconeogenesis must occur to meet the basal requirements of the body for glucose and to maintain the intermediates of the citric acid cycle. • Gluconeogenesis effectively clears metabolites such as lactate, glycerol, propionate etc from the blood.
- 36. KEY GLUCONEOGENIC ENZYMES • The irreversible steps in glycolysis are circumvented by four enzymes which are designated as the key enzymes of gluconeogenesis. 1. Pyruvate carboxylase 2. Phosphoenolpyruvate carboxykinase 3. Fructose-1,6- bisphosphatase 4. Glucose-6- phosphatase Fig. Carbohydrate synthesis from simple precursors
- 37. Reactions of gluconeogenesis • Gluconeogenesis and glycolysis are not identical pathways running in opposite direction, although they do share several steps; seven of the ten enzymatic reactions of gluconeogenesis are the reverse of glycolytic reactions. • The three reactions of glycolysis are essentially irreversible in vivo and cannot be used in gluconeogenesis and are bypassed by a set of separate enzymes. 1. Phosphoenolpyruvate is formed from pyruvate: 2. Fructose 6-phosphate is formed from fructose 1,6-bisphosphate: 3. Glucose is formed by hydrolysis of glucose 6-phosphate:
- 38. Bypass reaction of gluconeogenesis 1. Conversion of pyruvate to phosphoenolpyruvate • The first of the bypass reactions in gluconeogenesis is the conversion of pyruvate to phosphoenolpyruvate (PEP). • This reaction cannot occur by reversal of the pyruvate kinase reaction of glycolysis as it is irreversible under the conditions prevailing in intact cells. • Instead, the phosphorylation or pyruvate is achieved by a roundabout sequence of reactions that in eukaryotes requires enzymes in both the cytosol and mitochondria. When pyruvate or alanine is the glucogenic precursor: • Pyruvate is first transported from cytosol into mitochondria or is generated from alanine within mitochondria by transamination. • Then pyruvate carboxylase, a mitochondrial enzyme that requires the coenzyme biotin, converts the pyruvate to oxaloacetate. • The reaction involves biotin as a carrier of activated HCO3 - • The pyruvate carboxylase reaction can replenish intermediates in another central metabolic pathway, the citric acid cycle. • Because the mitochondrial membrane has no transporter for oxaloacetate, before export to the cytosol the oxaloacetate formed from pyruvate must be reduced to malate by mitochondrial malate dehydrogenase, at the expense of NADH.
- 39. • Mitochondrial malate dehyrogenase functions in both gluconeogenesis and the citric acid cycle, but the flow of metabolites in the two processes is in opposite directions. • Malate leaves the mitochondrion through a specific transporter in the inner mitochondrial membrane and in the cytosol it is reoxidized to oxaloacetate, with the production of cytosolic NADH. • The oxaloacetate is then converted to PEP by phosphoenolpyruvate carboxykinase. This Mg2+ dependent reaction requires GTP as the phosphoryl group donor. • Two high energy phosphate equivalents (one from ATP and one from GTP), must be expensed to phosphorylate one molecule of pyruvate to PEP. In contrast, when PEP is converted to pyruvate during glycolysis, only one ATP is generated from ADP. • The CO2 added to pyruvate in the pyruvate carboxylase step is the same molecule that is lost in the PEP carboxykinase reaction. • There is a logic to the route of these reactions through the mitochondrion. • The [NADH]/ [NAD+] ratio in the cytosol is 8 x 10-4, about 105 times lower than in mitochondria. Because cytosolic NADH is consumed in gluconeogenesis (in the conversion of 1,3-bisphosphoglycerate to glyceraldehyde 3-phosphate, glucose biosynthesis cannot proceed unless NADH is available.
- 40. • The transport of malate from the mitochondrion to the cytosol and its reconversion there to oxaloacetate effictively moves reducing equivalents to the cytosol, where they are scare. • This path from pyruvate to PEP therefore provides an important balance between NADH produced and consumed in the cytosol during gluconeogenesis. When lactate is the glucogenic precursor: • A second pyruvate to PEP bypass predominates when lactate is the glucogenic precursor. • This pathway makes use of lactate produced by glycolysis in erythrocytes or anaerobic muscle, for example, and it is particularly important in large vertebrates after vigorous exercise. • The conversion of lactate to pyruvate in the cytosol of hepatocytes yields NADH, and the export of reducing equivalents ( as malate) from mitiochondria is therefore unnecessary. • After the pyruvate produced by the lactate dehydrogenase reaction is transported into the mitochondrion, it is converted to oxaloacetate by pyruvate carboxylase. • This oxaloacetate, however, is converted directly to PEP by a mitochondrial isoenzyme of PEP carboxykinase, and the PEP is transported out the mitochondrion to continue on the gluconeogenic path. • The mitochondrial and cytosolic isoenzymes of PEP carboxykinase are encoded by separate genes in the nuclear chromosomes, providing another example of the two distinct enzymes catalyzing the same reaction but having different cellular locations or metabolic roles.
- 41. Fig. Alternative paths from pytuvate to phosphoenolpyruvate
- 42. 2. Conversion of fructose 1,6-Bisphosphate to Fructose 6- phospahte is the second bypass • The second glycolytic reaction that cannot participate in gluconeogenesis is the phosphorylation of fructose 6-phosphate by PFK-1. • As this reaction is irreversible in intact cells, the generation of fructose 6-phosphate from fructose 1,6-bisphospahte is catalyzed by a different enzyme, Mg2+ - dependent fructose 1,6-bisphosphatase (FBPase-1), which promotes the essentially irreversible hydrolysis of the C-1 phosphate (not phosphoryl group transfer to ADP). 3. Conversion of glucose6-phosphate to glucose is the third bypass • The third bypass is the final reaction of gluconeogenesis, the dephosphorylation of glucose 6-phosphate to yield glucose. • Reversal of the hexokinase reaction would require phosphoryl group transfer from glucose 6-phosphate to ADP, forming ATP, an energetically unfavorable reaction. • The reaction catalyzed by glucose 6-phosphatase does not require synthesis of ATP; it is a simple hydrolysis of a phosphate ester. • This mg2+ - activated enzyme is found on the lumenal side of the endoplasmic reticulum of hepatocytes and renal cells. • Muscle and brain tissue do not contain this enzyme and so cannot carry out gluconeogenesis. • Glucose produced by gluconeogenesis in the liver or kidney or ingested in the diet is delivered to brain and muscle through blood stream.
- 44. Energetics of gluconeogenesis • Gluconeogenesis is energetically expensive but essential process. • For each molecule of glucose formed from pyruvate, six high energy phosphate groups are required. a. Four from ATP b. Two from GTP c. In addition two molecules of NADH are required for the reduction of two molecules of 1,3-bisphosphoglycerate. • Conversion of glucose to pyruvate by glycolysis would require only two molecules of ATP. • So 3 ATPs are used by 1 pyruvate residue to produce one-half molecule of glucose: or 6 ATPs are required to generate one glucose molecule.
- 45. Regulation of gluconeogenesis Coordinated regulation of glycolysis and gluconeogenesis • If glycolysis (the conversion of glucose to pyruvate) and gluconeogenesis (the conversion of pyruvate to glucose) were allowed to proceed simultaneously at high rates, the result would be the consumption of ATP and the production of heat i.e. hydrolysis of ATP without any useful metabolic work being done. Therefore, gluconeogenesis and glycolysis are reciprocally regulated so that one pathway is relatively inactive when the other is active.
- 46. • Gluconeogenesis is regulated by the enzymes which catalyze and bypass irreversible steps of glycolysis, i.e. pyruvate carboxylase, PEP carboxykinase, fructose-1,6- bisphosphatase and glucose-6- phosphatase. • Turning-on gluconeogenesis is thus accomplished by shutting-off glycolysis. Fig. Reciprocal regulation of gluconeogenesis and glycolysis in the liver
- 47. 1. Pyruvate carboxylase • It is an allosteric enzyme. • Acetyl CoA is an activator of pyruvate carboxylase so that generation of oxaloacetate is favoured when acetyl CoA is sufficiently high. 2. Fructose-1,6-bisphosphatase • Citrate is an activator while fructose-2,6-bisphosphate and AMP are inhibitors. • All these three effectors have an exactly opposite effect on the phosphofructokinase (PFK). 3. ATP • Gluconeogenesis is enhanced by ATP. 3. Hormonal regulation of gluconeogenesis • The hormones glucagon and glucocorticoids increase gluconeogenesis. • Glucocorticoids induce the synthesis of hepatic amino transferases thereby providing substrate for gluconeogenesis. • The high glucagon-insulin ratio also favors induction of synthesis of gluconeogenic enzymes (PEPCK, fructose-1,6-bisphosphatase and glucose-6- phosphatase)
- 48. GLUCONEOGENESIS FROM AMINO ACIDS • Glucogenic amino acids are alanine, glutamic acid, aspartic acid etc. • When glucose is not readily available (starvation or diabetes mellitus), the glucogenic amino acids are transaminated to corresponding carbon skeletons. • These then enter the TCA cycle and form oxaloacetate or pyruvate. • Alanine released from the muscle is the major substrate for gluconeogenesis. Muscle wastage seen in uncontrolled diabetes mellitus could be explained by this factor.
- 49. GLUCONEOGENESIS FROM GLYCEROL • Glycerol is liberated mostly in the adipose tissue by the hydrolysis of fats (triacylglycerols). • The enzyme glycerokinase (found in liver and kidney, absent in adipose tissue) activates glycerol to glycerol 3-phosphate. • It is then oxidized to dihydroxyacetone phosphate by NAD+ dependent dehydrogenase.
- 50. GLUCONEOGENESIS FROM PROPIONATE • Propionyl CoA is formed from odd chain fatty acids and carbon skeleton of some amino acids. • It is converted to succinyl CoA which enters gluconeogenesis via citric acid cycle and is a minor source for glucose. • Even chain fatty acids cannot be converted to glucose; they are not substrates for gluconeogenesis.
- 51. ALCOHOL INHIBITS GLUCONEOGENESIS • Ethanol has been a part of the human diet for centuries. • Ethanol cannot be excreted and must be metabolized, primarily by the liver. • Ethanol oxidation in the liver to acetaldehyde by the enzyme alcohol dehydrogenase utilizes NAD+. Therefore, ethanol consumption leads to an accumulation of NADH. • This high concentration of NADH inhibits gluconeogenesis by preventing the oxidation of lactate to pyruvate. • In fact, the high concentration of NADH will cause the reverse reaction to predominate, and lactate will accumulate. The consequences may be hypoglycemia and lactic acidosis. • The overabundance of NADH also inhibits fatty acid oxidation. • The metabolic purpose of fatty acid oxidation is to generate NADH for ATP generation by oxidative phosphorylation, but an alcohol consumer’s NADH needs are met by ethanol metabolism. • In fact, the excess NADH signals that signals are right for fatty acid synthesis. Hence, triacylglycerols accumulate in the liver, leading to a condition known as “fatty liver”.
- 52. Fig. metabolism of ethanol
- 53. Why conversion of fat (acetyl-CoA) to glucose is not possible in human being? • Conversion of acetyl-CoA to glucose is not possible because of following reasons: 1. The pyruvate dehydrogenase reaction is essentially non- reversible which prevents the direct conversion of acetyl-CoA to pyruvate. 2. Secondly, there cannot be a net conversion of acetyl-CoA to oxaloacetate via citric acid cycle, since one molecule of oxaloacetate is consumed to condense with acetyl-CoA and only one molecule of oxaloacetate is regenerated, which is not formed De Novo (new) when acetyl-CoA is oxidized by citric acid cycle.
- 54. GLYCOGEN METABOLISM INTRODUCTION • Glycogen is the storage form of glucose in animals, as is starch in plants. • The main stores of glycogen in the body are found in skeletal muscle and liver. • The glycogen content of liver (10g/100g tissue) is more than in skeletal muscle (1-2g/100g). • Due to more muscle mass, the quantity of glycogen in muscle (250g) is about three times higher than that in the liver (75g). • Glycogen is stored as granules in the cytosol. FUNCTIONS OF GLYCOGEN • When blood glucose level lowers, liver glycogen is broken down and helps to maintain blood glucose level. • After taking food, blood sugar tends to rise, which causes glycogen deposition in liver. • About 5 hours after taking food, the blood sugar tends to fall. But, glycogen is lysed to glucose so that the energy needs are met. • The function of muscle glycogen is to act as reserve fuel for muscle contraction.
- 55. WHY STORE GLYCOGEN AS A FUEL RESERVE? GLYCOGEN • Glycogen can be rapidly mobilized. • Glycogen can generate energy in the absence of oxygen. • Brain depends on continuous glucose supply (which mostly comes from glycogen). FAT • Fat mobilization is slow. • Needs oxygen for energy production. • Cannot produce glucose (to a significant extent)
- 56. STRUCTURE OF GLYCOGEN • Glycogen is a branched-chain polysaccharide made exclusively from α-D-glucose. • The primary glycosidic bond is an α(1 4) linkage. • After an average of eight to ten glucosyl residues, there is a branch containing an α(1 6) linkage. • A single molecule of glycogen can have a molecular mass of up to 108 daltons. • These molecules exist in discrete cytoplasmic granules that also contain most of the enzymes necessary for glycogen synthesis and degradation.
- 57. GLYCOGEN METABOLISM IN LIVER VERSUS MUSCLE Parameter Liver Skeletal muscle Glycogen concentration Higher: 10% w/w Lower: 2% w/w Absolute amount of glycogen in healthy adults in well-fed state Lower: ~70g Higher: ~250g because muscle mass (~35 kg) is much greater than liver mass (1.8 kg). Glucose-6-phosphatase Present, hence free glucose is released after glycogenolysis Absent, hence glucose-6- phosphatase formed in glycogenolysis directly enters glycolysis in the muscle itself. Hormonal regulation of glycogenolysis More responsive to glucagon More responsive to epinephrine Purpose of glycogenolysis To regulate blood glucose levels in between meals, for use by brain, muscles, RBC To provide glucose for its own use
- 58. GLYCOGENOLYSIS DEFINITION • Glycogenolysis is the breakdown of glycogen to glucose or glucose-6- phosphate. • Glucose is the product of glycogenolysis in the liver because of the presence of enzyme glucose 6-phosphatase. • Glucose 6-phosphate is the end product of glycogenolysis in the muscle because of the absence of the enzyme, glucose 6-phosphatase. TISSUES ACTIVE IN GLYCOGENOLYSIS • Tissues that are mainly active in glycogenolysis are liver, skeletal muscle and to a minor extent the brain, kidney and intestine. SITE • Glycogenolysis is cytosolic processes.
- 59. 1. Action of glycogen phosphorylase • Glycogen phosphorylase catalyzes the reaction in which an (α1→4) glycosidic linkage between two glucose residues at a non-reducing end of glycogen undergoes attack by inorganic phosphate (Pi), removing the terminal glucose residue as α-D-glucose-1-phosphate. • This phosphorolysis reaction is different from the hydrolysis of glycosidic bonds by amylase during intestinal degradation of dietary glycogen and starch. • In phosphorolysis, some of the energy of the glycosidic bond is preserved in the formation of the phosphate ester, glucose-1-phosphate. • Pyridoxal phosphate is an essential cofactor in the glycogen phosphorylase reaction; its phosphate group acts as a general acid catalyst, promoting attack by Pi on the glycosidic bond. • Glycogen phosphorylase acts repetitively on the non- reducing ends of glycogen branches until it reaches a point four glucose residues away from an (α1→6) branch point, where its action stops. • If glycogen phosphorylase alone acts on a glycogen molecule, the final product is a highly branched molecule, it is called as limit dextrin.
- 60. 2. Action of debranching enzyme • Further degradation by glycogen phosphorylase can occur only after the debranching enzyme, formally known as oligo (α1→6) to (α1→4) glucantransferase, catalyzes two successive reactions that transfer branches. • Debranching enzyme has two enzyme activities present on a single polypeptide, hence it is a bifunctional enzyme. • α 1-4α 1-6 glucan transerase component, transfers the outer three residues from a branch (containing 4 glucosyl units) to a free C-4 of glucose of second branch point. • α1-6 glycosidase component hydrolyzes α (1-6) glycosidic bond at the branch point and releases free glucose. • Once these branches are transferred and the glucosyl residue at C-6 is hydrolyzed, glycogen phosphorylase activity can continue.
- 61. 3. Formation of glucose 6-phospahte and glucose • Glucose 1-phosphate, the end product of the glycogen phosphorylase reaction, is converted to glucose-6-phosphate by phosphoglucomutase, which catalyzed the reversible reaction. • The glucose 6-phosphate formed from glycogen in skeletal muscle can enter glycolysis and serve as an energy source to support muscle contraction. • In liver, glycogen breakdown serves a different purpose: to release glucose into the blood when the blood glucose level drops, as it does between meals. • This requires an enzyme, glucose 6- phosphatase that is present in liver and kidney but not in other tissues.
- 62. • The enzyme is an integral membrane protein of the endoplasmic reticulum, predicted to contain nine transmembrane helices, with its active site on the lumenal side of the ER. • Glucose 6-phosphate formed in the cytosol is transported into the ER lumen by a specific transporter (T1) and hydrolyzed at the lumenal surface by the glucose 6-phosphatase. • The resulting Pi and glucose are thought to be carried back into the cytosol by two different transporters (T2 and T3), and the glucose leaves the hepatocyte via yet another transporter in the plasma membrane (GLUT2). • By having the active site of glucose 6- phosphate inside the ER lumen, the cell separates this reaction form the process of glycolysis, which takes place in the cytosol.
- 63. Fig. Overview of Glycogenolysis
- 64. Role of sugar nucleotides in biosynthesis • Many of the reactions in which hexoses are transformed or polymerized involve sugar nucleotides, compounds in which the anomeric carbon of a sugar is activated by attachment to a nucleotide through a phosphate ester linkage. • Sugar nucleotides are the substrates for polymerization of monosaccharides into disaccharides, glycogen, starch, cellulose and more complex extracellular polysaccharides. • They are also key intermediates in the production of the aminohexoses and deoxyhexoses found in some of these polysaccharides, and in the synthesis of vitamin C (L-ascorbic acid). • The role of sugar nucleotides in the biosynthesis of glycogen and many other carbohydrate derivatives was first discovered by the Argentine biochemist Luis Leloir.
- 65. • The suitability of sugar nucleotides for biosynthetic reactions stems from several properties: 1. Their formation is metabolically irreversible, contributing to the irreversibility of the synthetic pathways in which they are intermediates. 2. Although the chemical transformation of sugar nucleotides do not involve the atoms of the nucleotide itself, the nucleotide moiety has many groups that can undergo noncovalent interactions with enzyme; the additional free energy of binding can contribute significantly to catalytic activity. 3. Like phosphate, the nucleotidyl group (UMP or AMP, for example) is an excellent leaving group, facilitating nucleophilic attack by activating the sugar carbon to which it is attached. 4. By “tagging” some hexoses with nucleotidyl groups, cells can set them aside in a pool for one purpose (glycogen synthesis, for example), separate from hexose phosphates destined for another purpose (such as glycolysis). Fig. Formation of a sugar nucleotide. A condensation reaction occurs between nucleoside triphosphate (NTP) and a sugar phosphate. The negatively charged oxygen on the sugar phosphate serves as a nucleophile, attacking the alpha phosphate of the nucleoside triphosphate and displacing pyrophosphate. The reaction is pulled in the forward direction by the hydrolysis of PPi by inorganic pyrophosphatase.
- 66. GLYCOGENESIS DEFINITION • Glycogenesis is the biosynthesis of glycogen from glucose. TISSUES ACTIVE IN GLYCOGENESIS • The major tissues synthesizing glycogen are liver and muscle. • Small amounts of glycogen are also synthesized in brain and small intestine. SITE • Glycogenesis is cytosolic processes, occurs in the fed state, when insulin/glucagon ratio is high. REQUIREMENTS • ATP and UTP beside glucose.
- 67. REACTIONS 1. Formation of glucose 6- phosphate • The starting point for synthesis of glycogen is glucose 6-phosphate which can be derived from free glucose in a reaction catalyzed by the isoenzyme hexokinase I and hexokinase II in muscle and hexokinase IV (glucokinase) in liver. • The cofactors required are ATP and Mg2+ . 2. Formation of glucose 1- phosphate • To initiate glycogen synthesis, the glucose 6-phosphate is converted to glucose 1-phosphate. • The reaction is catalyzed by phosphoglucomutase.
- 68. 3. Formation of UDP- Glucose • UDP-glucose (uridine diphosphoglucose) is formed from the interaction of UTP and glucose 1-phosphate. • The reaction is catalyzed by the enzyme, UDP-glucose pyrophosphorylase. • This enzyme is named for the reverse reaction; in the cell, the reaction proceeds in the direction of UDP- glucose formation. • The pyrophosphate so released is immediately hydrolyzed to two molecules of inorganic phosphate by the enzyme pyrophosphatase.
- 69. 4. Formation of linear polymer of glycogen • UDP-glucose is the immediate donor of glucose residues. • Glycogen is synthesized by the addition of glucose units from UDP glucose to a glycogen primer. • The reaction is catalyzed by the enzyme, glycogen synthase. • Glycogen synthase transfers glucosyl residues from UDP glucose to the C-4 hydroxyl group at the non-reducing end of the glycogen molecule through a α1-4 glycosidic bond. • Glycogen synthase cannot initiate a new glycogen chain de novo. • It requires a primer, usually a preformed (α1-4 ) polyglucose chain or branch having at least eight glucose residues. • The intriguing protein glycogenin is both the primer on which new chains are assembled and the enzyme that catalyzes their assembly. • The first step in the synthesis of a new glycogen molecule is the transfer of a glucose residues from UDP-glucose to the hydroxyl group of Tyr194 of glycogenin, catalyzed by the protein’s intrinsic glycosyltransferase activity.
- 70. • The nascent chain is extended by the sequential addition of seven more glucose residues, each derived form UDP-glucose; the reactions are catalyzed by the chain-extending activity of glycogenin. • At this point, glycogen synthase takes over, further extending the glycogen. • Glycogenin remains buried within the particle, covalently attached to the single reducing end of the glycogen molecule.
- 71. 5. Formation of branched polymer of glycogen • Glycogen synthase cannot make the (α 1 →6) bonds found at the branch points of glycogen; these are formed by the glycogen-branching enzyme, also called amylo 1-4,1-6-transglycosylase or glycosyl (4 →6) transferase . • The glycogen branching enzyme catalyzes transfer of a terminal fragment of 6 or 7 glucose residues from the non-reducing end of a glycogen branch having at least 11 residues to the C-6 hydroxyl group of a glucose residue that is at least 4 residues away from the branch point at a more interior position of the same or another glycogen chain, thus creating a new branch. • Further glucose residues may be added to the new branch by glycogen synthase.
- 72. • The branching of glycogen is essential in two ways: 1. Branches permit the storage of glycogen as a compact molecule, and 2. Make the glycogen molecule more soluble and to increase the number of reducing ends. This increases the number of sites accessible to glycogen phosphorylase and glycogen synthase, both of which act only at non-reducing ends.
- 74. Regulation of glycogen metabolism A good coordination and regulation of glycogen synthesis and its degradation are essential to maintain the blood glucose levels. Glycogenesis and glycogenolysis are, respectively, controlled by the enzymes glycogen synthase and glycogen phosphorylase. Regulation of these enzymes is accomplished by three mechanisms 1. Allosteric regulation 2. Hormonal regulation 3. Influence of calcium Allosteric regulation of glycogen metabolism • There are certain metabolites that allosterically regulate the activities of glycogen synthase and glycogen phosphorylase. • The control is carried out in such a way that glycogen synthesis is increased when substrate availability and energy levels are high. • On the other hand, glycogen breakdown is enhanced when glucose concentration and energy levels are low. • In a well-fed state, the availability of glucose 6- phosphate is high which allosterically activates glycogen synthase for more glycogen synthesis. • On the other hand, glucose 6-phosphate and ATP allosterically inhibit glycogen phosphorylase. • Free glucose in liver also acts as an allosteric inhibitor of glycogen phosphorylase. Fig. Allosteric regulation of glycogen synthesis and degradation in A. Liver and B. Muscle
- 75. Hormonal regulation of glycogen metabolism • The hormones, through a complex series of reactions, bring about covalent modification, namely phosphorylation and dephosphorylation of enzyme proteins which, ultimately control glycogen synthesis or its degradation.
- 76. Fig. Hormonal regulation of glycogen degradation (glycogenolysis)
- 77. Effect of calcium on glycogenolysis • When the muscle contracts, Ca2+ ions are released from the sarcoplasmic reticulum. • Ca2+ binds to calmodulin- calcium modulating protein and directly activates phosphorylase kinase without the involvement of cAMP- dependent protein kinase.
- 78. GLYCOGEN STORAGE DISEASES • Glycogen storage diseases are a group of inherited disorders associated with glycogen metabolism where an abnormal type or quantity of glycogen is deposited in different tissues. • According to the deficiency of the enzyme involved, there are several types of glycogen storage diseases. • The different forms are categorized by numerical number (type), which is given in a chronological sequence in which the disorders were identified. • All these are autosomal recessive disorders except Her’s disease which is autosomal dominant.
- 79. Rare glycogen disorders IX, X and XI have been identified. They are due to defects in the enzymes concerned with activating and deactivating liver phosphorylase.
- 80. CASE STUDY • The patient was a 12-year old girl who had a grossly enlarged abdomen. She had a history of frequent episodes of weakness, sweating and pallor that were eliminated by eating. Her development had been slow; she sat at the age of 1 year, walked unassisted at the age of 2 years, and was doing poorly in the school. Physical examination revealed normal blood pressure, temperature and a normal pulse rate but a subnormal weight (23 kg). The liver was enlarged, firm and was descended in to pelvis. The spleen was not palpable, nor were the kidneys. The remainder of the physical examination was within the normal limits. laboratory investigation reports revealed, low blood glucose, low pH, high lactate, triglycerides, ketones and high free fatty acids. The liver biopsy revealed high glycogen content. Hepatic glycogen structure was normal. The enzyme assay performed on the biopsy tissue revealed very low glucose-6-phosphatase levels. 1. What is the probable diagnosis? 2. What is the possible treatment for this patient?
- 81. CASE DETAILS • The girl is suffering from von Gierke’s disease. The clinical picture, biochemical findings, hypoglycemia and increased hepatic glycogen stores are all characteristics of von Gierke’s disease. Von Gierke’s disease • Glycogen storage disease (GSD) Type I, is also known as von Gierke’s disease or hepatorenal glycogenesis. Von Gierke’s described the first patient with GSD Type I in 1929 under the name hepatonephromegalia glycogenica. GSD Type I is divided into GSD Type Ia caused by G6Pase deficiency and GSD Ib resulting from deficiency of a specific translocase T1. Pathophysiology of von Gierke’s disease • Because of insufficient G6Pase activity, G6P cannot be converted to free glucose, but G6P is metabolized to lactic acid or incorporated into glycogen. In this way, large quantities of glycogen are formed and stored as lactic acid or incorporated into glycogen. In this way, large quantities of glycogen are formed and stored as molecules with normal structure in the cytoplasm of hepatocytes and renal and intestinal mucosa cells, therefore, enlarged liver and kidneys dominate the clinical presentation of the disease. The chief biochemical alteration is hypoglycemia, while secondary abnormalities are hyperlactemia, metabolic acidosis, hyperlipidemia, and hyperuricemia.
- 82. • In hypoglycemia, the deficiency of G6Pase blocks the process of glycogen degradation and gluconeogenesis in the liver, preventing the production of free glucose molecules. As a consequence, patients with GSD Type I have fasting hypoglycemia. Despite the metabolic block, the endogenous glucose formation is not fully inhibited in young patients, production of free glucose reaches half that of healthy individuals, whereas adult patients may produce as much as two thirds of the healthy amount of free glucose. Hypoglycemia inhibits insulin secretion and stimulates glucagon and cortisol release. • In hyperlactatemia and acidosis, undegraded G6P, galactose, fructose and glycerol are metabolized via the G6P pathway to lactate, which is used in the brain as an alternative source of energy. The elevated blood lactate levels cause metabolic acidosis. • In hyperuricemia, blood uric acid levels are raised because of the increased endogenous production and reduced urinary elimination caused by competition with the elevated concentration of lactate, which should be excreted. • In hyperlipidemia, elevated endogenous triglyceride synthesis from nicotinamide adenine dinucleotide (NADH), NAD phosphate (NADPH), acetyl-coenzyme A (CoA), glycerol, and diminished lipolysis causes hyperlipidemia. Triglycerides increase the risk of fatty liver infiltration, which contributes to the enormous amount of liver enlargement. Despite significantly elevated serum triglycerides levels in patients, vascular lesions and atherosclerosis are rare complications. The increased serum Apolipoprotein E concentration that promote the clearance of triglycerides may explain the rarity of such complicaitons.
- 83. Fig. An overview of glycogen metabolism
- 84. Incidence • Patients with glycogen storage disease (GSD) Type I account for 24.6 percent of all patients with GSD. • Based on the results of combined US and European studies, the cumulative incidence is estimated at 1 in 20,000 to 25,000 live births. Inheritance • Type I glycogen storage disease is an autosomal recessive disorder. As with other genetically determined diseases, GSD Type I develops during conception, yet the first signs of the disease may appear at birth or later. Clinical manifestations • The earliest sign of the disease may develop shortly after birth and are caused by hypoglycemia and lactic acidosis. • Convulsions are a leading sign of disease. • Frequent symptoms of moderate hypoglycemia, such as irritability, pallor, cyanosis, hypotonia, tremors, loss of consciousness, and apnea, are present. • A leading sign of GSD Type I is enlargement of the liver and kidneys. During the first weeks of life, the liver is normal size. It enlarges gradually thereafter, and in some patients, it even reaches the symphysis.
- 85. • The patient’s face is characteristically reminiscent of a doll’s face (rounded cheeks due to fat deposition). • Mental development proceeds normally. • Growth is retarded, and children affected with GSD Type I never gain the height otherwise expected from the genetically determined potential of their families. The patient’s height is usually below the third percentile for their age. The onset of puberty is delayed. • Late complication of disease are renal function disturbance (including nephrocalcinosis), renal stones, tubular defects, and hypertension, mainly in patients older than 20 years of age. • Skin and mucous membrane changes include the following: - Eruptive xanthomas develop on the extensor surfaces of the extremeties. - Tophy or gouty arthritis may occur. - Many patients bleed easily particularly from the nose. This tendency is a result of altered platelet function due to the platelets lower adhesiveness.
- 86. Laboratory investigations • Serum glucose and blood pH levels are frequently decreased, while the serum lactate, uric acid, triglyceride, and cholesterol levels are elevated. Urea and creatinine levels might be elevated when renal function is impaired. • The following laboratory values should be obtained: - Serum glucose and electrolyte levels (higher anion gap may suggest lactic acidosis) - Serum lactate level - Blood pH - Serum uric acid level - Serum triglyceride and cholesterol levels - Gamma glutamyltransferase level - CBC and differential (e.g. anemia, leucopenia, neutropenia) - Coagulation - bleeding and clotting time - Urinalysis for aminoaciduria, proteinuria, and microalbuminuria in older patients - Urinary excretion levels of uric acid calcium - Serum alkaline phosphatase, calcium, phosphorous, urea and creatinine levels
- 87. Imaging studies • In GSD Type I, liver and kidney ultrasonography should be performed for follow-up of organomegaly and detection of hepatic adenomas and nephrocalcinosis. • Other tests • Glucagon and epinephrine tests do not cause a rise in glucose levels, but plasma levels of lactic acid are raised. • Orally administered galactose and fructose (1.75 g/kg PO) progressively lowers lactic acid levels over several hours after administration of glucose. Treatment • Because no specific treatment is available, symptomatic therapy is very important. • The primary goal of treatment is to correct hypoglycemia and maintain a normoglycemic state. The normoglycemic state can be achieved with overnight nasogastric infusion of glucose, parenteral nutrition, or per oral adminstration of raw cornstarch. Glucose molecules are continuously released by hydrolysis of raw cornstarch in the digestive tract over 4 hours following its intake. • The intake of fructose and galactose should be restricted because it has been shown that they cannot be converted to glucose but that they do increase lactate production.
- 88. Medication • No specific drug treatment is recommended for GSD Type I. • Allopurinol (Zyloprim), a xanthine oxidase inhibitor can reduce uric acid levels in the blood and prevent occurrence of gout and kidney stones in adult life. • Hyperlipidemia can be reduced by lipid-lowering drugs (e.g. 3-hydroxy-3- methylglutaryl coenzyme A reductase inhibitors, fibric acid derivatives). • In patients with renal lesions, microalbuminuria can be reduced with angiotensin-converting enzyme (ACE) inhibition therapy. • Nephrocalcinosis and renal calculi can be prevented with citrate therapy. • Additionally, for patients with GSD Type I, the future may bring Adeno- associated virus vector-mediated gene therapy, which may result in curative therapy. Mortality/Morbidity • In GSD Type I, acute hypoglycemia may be fatal. Early death is now uncommon with improving care and treatment. • Late complication, such as renal failure, hypertension, or malignant alteration of hepatic adenomas, may be responsible for mortality in adolescent and adult patients.
- 89. HMP SHUNT PATHWAY DEFINITION • Hexose monophosphate shunt (HMP shunt) or pentose phosphate pathway or phosphogluconate pathway is the second major pathway for the metabolism of glucose. • It is an alternate pathway to glycolysis and TCA cycle for the glucose oxidation leading to the production of NADPH and pentose sugars. • Pentose phosphate pathway is not involved in the generation of energy. TISSUES ACTIVE IN PENTOSE PHOSPHATE PATHWAY • HMP shunt pathway is operative in many tissues, such as the liver, erythrocytes, lactating mammary gland, testes and the adipose tissue. • Tissues most enriched in enzymes of pentose phosphate pathway are those that have the greater demand for NADPH. • Rapidly dividing cells, such as those of bone marrow, skin and intestinal mucosa, use the pentoses to make RNA, DNA and such coenzymes as ATP, NADH, FADH2, and coenzyme A. SITE • The reactions of pentose phosphate pathway occur in the cytosol.
- 91. Overview of pentose phosphate pathway
- 92. REACTIONS OF PENTOSE PHOSPHATE PATHWAY • The reactions of the pathway are divided into two phases: 1. Phase I : Oxidative irreversible phase 2. Phase II : Non-oxidative reversible phase THE OVERALL PROCESS OF HMP SHUNT • In the overall process, six molecules of hexoses (glucose 6-phosphate) are utilized where one molecule is oxidized as 5-molecules are finally recovered. • 6 Glucose 6-phosphate + 12 NADP+ + 6H2O → 6CO2 + 12 NADPH + 12H+ + 5 Glucose 6-phosphate
- 93. Oxidative reactions of the pentose phosphate pathway • In some tissues, the pentose phosphate pathway ends at this point, and its overall equation is Glucose 6-phosphate + 2NADP+ + H2O → ribose 5-phosphate + CO2 + 2NADPH + 2H+ • The net result is the production of NADPH, a reductant for biosynthetic reactions, and ribose 5- phosphate, a precursor for nucleotide synthesis.
- 94. Non-oxidative reactions of the pentose phosphate pathway
- 95. REGULATION OF PENTOSE PHOSPHATE PATHWAY • The first step of the pathway, catalyzed by glucose-6- phosphate dehydrogenase (G-6-PD) is the rate limiting step. • Glucose 6- phosphate dehydrogenase is regulated in two ways: 1. Allosteric regulation 2. Induction Induction (hormonal regulation) Glucose -6-phosphate dehydrogenase is an inducible enzyme. It is induced by insulin.
- 96. Allosteric regulation • Allosteric regulation is by cellular concentration of NADPH. • Supply of the substrate, NADP+ determines the rate of pentose phosphate pathway. • An increased concentration of NADPH decreases the activity of G-6-PD, for example: o Under, well fed condition the ratio of NADPH/NADP+ decreases and pentose phosphate pathway is stimulated. o In starvation and diabetes, the ratio of NADPH/NADP+ is high and inhibits the pathway.
- 97. SIGNIFICANCE OF HMP SHUNT • HMP shunt is unique in generating two important products – Pentoses and NADPH – needed for the biosynthetic reactions and other functions. Importance of pentoses • Rapidly dividing cells, such as those of bone marrow, skin and intestinal mucosa, and those of tumors, use the pentose ribose-5-phosphate for synthesis of RNA, DNA and coenzymes as ATP,NAD,FAD and Coenzyme A. Importance of NADPH • NADPH is required for the reductive biosynthesis of fatty acids and steroids. Hence HMP shunt is more active in the tissues concerned with lipogenesis, e.g. adipose tissue, liver etc. • NADPH is used in the synthesis of certain amino acids involving the enzyme glutamate dehydrogenase. • Microsomal cytochrome P450 system (in liver) brings about the detoxification of drugs and foreign compounds by hydroxylation reactions involving NADPH. • Phagocytosis is the engulfment of foreign particles including microorganisms, carried out by white blood cells. The process requires the supply of NADPH.
- 98. • NADPH produced in erythrocytes has special functions to perform. It maintains the concentration of reduced glutathione which is essentially required to preserve the integrity of RBC membrane. • NADPH is also necessary to keep the ferrous iron (Fe2+ ) of haemoglobin in reduced state so that accumulation of methehemoglobin (Fe3+ ) is prevented. • High concentration of NADPH in lens of eyes is necessary to preserve the transparency of the lens. • There is a continuous production of H2O2 in the living cells which can chemically damage unsaturated lipids, proteins and DNA. This is however prevented to a larger extent through antioxidant reactions involving NADPH.
- 99. CASE STUDY • A 34-year old African-American man was seen with fever and shortness of breath. Shortly afterwards he developed pancreatitis and was treated with an antibiotic, Clindamycin and Primaquine. After four days into this therapy the onset of hematuria was noted. The patient’s hemoglobin (Hb) fell from 11.0 g/dL to 7.4 g/dL, his total bilirubin increased from 1.2 mg/dL to 4.3 mg/dL and his lactic dehydrogenase (LDH) increased from 248 IU/L to 612 IU/L. 1. What is the probable diagnosis? 2. What is the relationship of primaquine and hemolytic anaemia?
- 100. Case details • The patient is most probably suffering from Glucose-6-phosphate dehydrogenase deficiency. The hemolysis is primaquine induced which is an oxidant drug. The rise in bilirubin is due to hemolytic jaundice and the rise in lactic dehydrogenase (LDH) is commonly observed in hemolytic anemias. Background • Glucose 6-phosphate dehydrogenase (G6PD) deficiency is the most common disease-producing enzymopathy in humans. Inherited as an X-linked disorder. The disease is highly polymorphic, with more than 300 reported variants. It confers protection against malaria, which probably accounts for its high gene frequency. Pathophysiology • Glucose 6-phosphate dehydrogenase (G6PD) is an enzyme critical in the redox metabolism of all aerobic cells. In red cells, its role is even more critical because it is the only source of reduced nicotinamide adenine dinucleotide phosphate (NADPH), which directly and via reduced glutathione (GSH), defends these cells against oxidative stress.
- 101. • Nicotinamide adenine dinucleotide phosphate (NADPH) is required cofactor in many biosynthetic reactions which also maintains glutathione in its reduced form. • Reduced glutathione acts as a scavenger for dangerous oxidative metabolites in the cell. With the help of enzyme glutathione peroxidase, reduced glutathione converts harmful hydrogen peroxide to water. • People deficient in G6PD are not prescribed oxidative drugs, because their red blood cells undergo rapid hemolysis under this stress. • Although in G6PD deficient subjects there is decrease in G6PD activity in most tissues, this is less marked than in red cells, and it does not seem to produce symptoms. Fig. Role of G-6PD in glucose metabolism
- 102. Incidence • The highest prevalence rates with gene frequencies from (5-25%) of glucose-6- phosphate dehydrogenase (G6PD) are found in tropical Africa, the Middle East, tropical and subtropical Asia and some areas of the Mediterrannean. • G6PD deficiency has been selected by plasmodium falciparum malaria, by virtue of the fact that it confers a relative resistance against this highly lethal infection. • Whether this protective effect is exerted mainly in hemizygous males or in females heterozygous for G6PD deficiency is still not clear. Inheritance • It is transmitted as X-linked recessive trait. Variants • There are several variants of the enzyme due to mutations in the gene coding for the enzyme. • Examples are 1. Variant GPD A (less severe and cause hemolytic anemia under conditions of oxidative stress) 2. Variant GPD B (more severe and cause hemolytic anemia in the absence of oxidative stress)
- 103. Clinical manifestations • The vast majority of people with G6PD deficiency remain clinically asymptomatic throughout their lifetime. • However, all of them have an increased risk of developing neonatal jaundice (NNJ) and a risk of developing acute hemolytic anemia when challenged by a number of oxidative agents. • NNJ related to G6PD deficiency is very rarely present at birth: the peak incidence of clinical onset is between day 2 and day 3, and in most cases the anemia is not severe. However, NNJ can be very severe in some G6PD deficient babies, especially in association with prematurity, infection, and/ or environmental factors. In these cases, if inadequately managed, NNJ associated with G6PD deficiency can produce Kernicterus and permanent neurologic damage. Precipitating factors • Acute hemolytic anemia (HA) can develop as a result of three types of triggers: (1) fava beans, (2) infections, and (3) drugs. • Typically, a hemolytic attack starts with malaise, weakness, and abdominal or lumbar pain. After an interval of several hours to 2 to 3 days, the patient develops jaundice and often dark urine, due to hemoglobinuria. • It is associated with hemoglobinemia, hemoglobinuria, and low or absent plasma Haptoglobin.
- 104. Drugs that precipitate the attack Definite risk Possible risk Doubtful risk Antimalarials Primaquine Chloroquine Quinine Sulphonamides/Sulp hones Sulphamethoxazole Sulfasalazine Sulfisoxazole Antibacterial/antibi otics Nalixidic acid Ciprofloxacin Norfloxacin Chloramphenicol Antipyretic/Analgesi cs Acetanilide Acetylsalicyclic acid (>3 g/day) Acetaminophen Other Naphthalene Vitamin K analogs Doxorubicin
- 105. Laboratory diagnosis • The laboratory workup for glucose-6-phosphate dehydrogenase (G6PD) deficiency includes the following: • Measure the actual enzyme activity of G6PD rather than the amount of glucose- 6-phosphate dehydrogenase. • Obtain a complete blood cell (CBC) count with the reticulocyte count to determine the level of anemia and bone marrow function. • Indirect bilirubin occurs with excessive hemoglobin degradation and can produce clinical jaundice. • Serum haptoglobin levels serve as an index of hemolysis and will be decreased. • LDH is high and so is the unconjugated bilirubin, indicating that there is also extravascular hemolysis. Imaging studies • Abdominal ultrasound may be useful in assessing for splenomegaly and gallstones in cases of glucose-6-phosphate dehydrogenase (G6PD) deficiency.
- 106. Treatment • Medical care : Identification and discontinuation of the precipitating agent is critical in cases of glucose-6-phosphate dehydrogenase (G6PD) deficiency. • Affected individuals are treated with oxygen and bed rest, which may afford symptomatic relief. • Prevention of drug-induced hemolysis is possible in most cases by choosing alternative drugs. • When acute HA develops and once its cause is recognized, no specific treatment is needed in most cases. • However, if the anemia is severe, it may be a medical emergency, especially in children, requiring immediate action, including blood transfusion. • If acute renal failure develops, hemodialysis may be necessary, but if there is no previous kidney disease, full recovery is the rule. • The management of NNJ associated with G6PD deficiency is no different from that of NNJ due to other causes. • Diet : patients must avoid broad beans (i.e. fava beans). Favism occurs only in the Mediterranean variety of glucose-6-phospahte dehydrogenase (G6PD) deficiency.
- 107. Fig. Mechanism of hemolysis in G6PD deficiency
- 108. THIAMIN DEFICIENCY Cause • Caused by the deficiency of thiamin in the diet. Features • The condition is known as beriberi. • Biochemical findings are (1) accumulation of pentose sugars in erythrocytes, and (2) decreased transketolase activity in erythrocytes. Diagnosis • The transketolase activity of RBC is used to measure nutritional status of thiamine. Treatment • Treatment is by administration of thiamine.
- 109. GALACTOSE METABOLISM SOURCE OF GALACTOSE • The disaccharide lactose present in milk and milk products, the principal dietary source of galactose. • Lactase (β-galactosidase) of intestinal mucosal cells hydrolyses lactose to galactose and glucose. • Galactose is also produced within the cells from the lysosomal degradation of glycoproteins and glycolipids. • As is the case for fructose, galactose entry into the cells is not dependent on insulin. SIGNIFICANCE OF GALACTOSE 1. METABOLISM OF GALACTOSE TO GLUCOSE • Galactose ( a component of lactose in milk) is converted to glucose in liver. Thus, galactose is an important dietary sugar in humans, who consume adequate amounts of milk. 2. METABOLISM OF UDP-GALACTOSE • During the metabolism of galactose, UDP-galactose is formed. UDP-galactose is utilized for (1)regeneration of UDP-glucose (2)synthesis of lactose (3) synthesis of glycoproteins (4) synthesis of glycosaminoglycans. SITE • The synthesis of glucose from galactose occurs mainly in the liver.
- 111. Fig. Conversion of galactose to UDP-glucose
- 112. Fig. Conversion of galactose to galactitol
- 113. CASE STUDY The patient, a boy, was the first child of healthy parents. Delivery was normal and birth weight was 3.8 Kgs. From the third day of life the child developed an increasing degree of jaundice and at the same time became indolent and difficult to feed. No blood group incompatibilty could be demonstrated. At 6 days of age he had a serum bilirubin of 14 mg/dL, and his weight was 15 percent below the normal weight. He was readmitted to the hospital on the seventh day after birth. Muscular tonus was increased, and the patient later began to convulse. Between the 7th day and 9th day, exchanged blood transfusion was performed three times, but the serum bilirubin concentration still remained high. On the 9th day of life the boy began vomiting, the liver enlargement was noted, and the cerebral symptoms became accentuated. A positive test for reducing sugar had already been obtained on the 6th day after birth. A repeated test performed on the 7th day was positive, whereas at the same time, a test specific for glucose was negative. Hereditary galactosemia was then suspected; special tests performed on the 8th day confirmed the diagnosis. Hb – 10.4 g/dL AST – 64 IU/L ALT – 40 IU/L Bilirubin – 14 mg/dL (at 7th day) Galactose-1phosphate-uridyl transferase – 0 (normal 2-31 units/g of Hb) (in RBC) 1. What are the biochemical effects of galactosemia ? 2. What are the interpretations of laboratory results ?
- 114. Case details • Hereditary galactosemia is among the most common carbohydrate metabolism disorders and can be a life-threatening illness during the newborn period. • Galctose-1-phosphate uridyl transferase (GALT) deficiency is the most common enzyme deficiency that causes galactosemia. • Removing lactose largely eliminates the toxicity associated with newborn disease, but long-term complications routinely occur. Fig. Metabolism of galactose and the entry of galactose carbon atoms into the glycolytic pathway
- 115. Pathophysiology • Galactosemia is associated with the following three enzyme deficiencies: • Galactokinase converts galactose to galactose-1-phosphate and is not a common deficiency. • Uridine diphosphate (UDP) galactose-4-epimerase epimerizes UDP galactose to UDP glucose and is also uncommon. • Deficiency of galactokinase causes cataracts. Deficiency of uridine diphosphate galactose-4-epimerase can be benign when the enzyme deficiency is limited to blood cells but can be as severe as classic galactosemia when the enzyme deficiency is generalized. • GALT is responsible for hereditary or classical galactosemia and is the most common deficiency. This enzyme catalyzes conversion of galactose-1- phosphate and UDP glucose to UDP galactose and glucose-1-phospahte. Individuals with GALT deficiency manifest abnormal galactose tolerance. Initially galactose-1-phosphate accumulates in the tissues and blood, as more and more of galactose and galactose-1-phosphate accumulate, damage to the cells occurs. In this case the major organ damaged by galactose accumulation is liver.
- 116. • Biosynthesis of galactitol : Galactitol is an alcohol that is made from galactose. The pathway is similar to that of formation of glucose. Because of the excessive quantity of galactose, some of it is converted to galactitol, which build-up in the body and is excessively excreted in urine. Since it is an alcohol and not a reducing substance. Therefore, the positive test for reducing substances in the urine is caused by galactose and not by galactitol. Galactitol appears to be toxic at high concentration and produces tissue injuries, especially cataract formation. Incidence • In United States incidence is approximately one case per 40,000 to 60,000 persons. The disorder is thought to be much less common in Asians. Genetic considerations • Classic galactosemia is caused by a severe deficiency in GALT. • The deficiency is an autosomal recessive genetic condition.
- 117. BIOCHEMICAL ABNORMALITIES AND FINDINGS • Galactose metabolism is impaired leading to increased galactose levels in circulation (galactosemia) and urine (galactosuria). • The accumulated galactose is diverted for the production of galactitol(dulcitol) by the enzyme aldose reductase. • Aldose reductase is present in lens, nervous tissue, seminal vesicles etc. • Galactitol is the causative factor for cataract. • The accumulation of galactose 1-phosphate and galactitol in various tissues like liver, nervous tissue, lens and kidney leads to impairment in their function. • High levels of galactose 1-phosphate in liver results in the depletion of inorganic phosphate (sequestering of phosphate) for other metabolic functions. • Galactose 1-phosphate inhibits glycogen phosphorylase resulting in hypoglycemia. Clinical manifestations • The cardinal features of classic glactosemia are hepatomegaly, catarcts and mental retardation. • Untreated infants with severely deficient galactose-1-phosphate uridyl transferase (GALT) activity typically present with the following variable finding:
- 118. • Poor growth within the first few weeks of life. • Jaundice • Bleeding from coagulopathy • Liver dysfunction and/or hepatomegaly • Cataracts (sometimes as early as the first few days of life) • Lethargy • Hypotonia • Sepsis (E.coli) • Blindness is due to the conversion of circulating galactose to the sugar alcohol galactitol, by an NADPH-dependent galactose reductase that is present in neural tissue an in the lens of the eye. At normal circulating levels of galactose this enzyme activity causes no pathological effects. However, a high concentration of galactitol in the lens causes osmotic swelling within the resultant formation of cataracts and other symptoms. • Learning problems, speech and language deficits are common. • The most common findings in adults include hypergonadotropic hypogonadism or primary ovarian insufficiency in women. • Short stature and neurologic abnormalities (e.g. tremor, ataxia and dystonia) also occur in a minority of patients.
- 119. Laboratory investigations 1. Urine test for reducing sugar is positive. The confirmation for the presence of galactose can be done by thin layer chromatograply. 2. Galactose intolerance test : a galactose tolerance test is abnormal in these patients. 3. Serum transaminases : both the serum transaminases are elevated suggesting the possibility of liver damage. 4. Serum bilirubin : It is elevated 5. Estimation of galactose-1-phosphate uridyl transferase : finally the diagnosis can be made demonstrating that the galactose-1-phosphate uridyl transferase is absent in the erythrocytes. Treatment • Treatment of galactosemia to prevent long-term complications remains a challenge. • The disease is treated by prescribing galactose free diet (removal of milk). • This diet causes most of the symptoms to regress except mental retardation.
Editor's Notes
- Topically : Of, relating to, or applied externally to a particular part of the body; local: a topical anesthetic. Gingivitis : Inflammation of the gums
- Necrosis : the death of cells, tissues or organs. Necrosis may be caused by numerous insults to tissues (e.g. insufficient blood supply, burns, comression, pathogenic microorganisms, medications, radiation, toxins, or tissue trauma).
- Sarcoma : A cancer arising from mesenchymal tissue such as muscle or bone, which may affect the bones, bladder, kidneys, liver, lungs, parotids, and spleen. Xenograft : A surgical graft of tissue from an individual of one species to an individual of a different species.
- Circumvent : to find a way of avoiding a difficulty or a rule; to go or travel around sth that is blocking your way.
- The reducing property is attributed to the free aldehyde or keto group of anomeric carbon.
- ATP consists of adenosine and is composed of an adenine ring, ribose sugar, and three phosphate groups (triphosphate). The groups of the phosphate group are usually called as the alpha (α), beta (β), and gamma (γ) phosphates. It is typically related to a monomer of RNA called adenosine nucleotide. Gamma phosphate group is the primary phosphate group on the ATP molecules that is hydrolyzed when the energy is needed to drive anabolic reactions. Basically gamma phosphate is typically located the farthest from the ribose sugar and has a higher energy of hydrolysis than either that of the alpha and beta phosphate. The bonds that are formed after hydrolysis or the phosphorylation of a residue by ATP are lower in energy than that of the phosphoanhydride bonds of ATP. The phosphoryl groups starting with that on AMP are referred to as the alpha, beta, and gamma phosphates.
- Intriguing : arousing one's curiosity or interest; fascinating.
- Grossly : visible to the nacked eye. Episodes : An occurrence that is one in a sequence of events. Pallor : Lack of color; paleness. Pulse : the normal resting pulse in adult is between 60 and 100 beats per minute. Temperature : Oral temperature is usually 97.5ͦ to 99.5ͦ F (36ͦ to 38ͦ C). Blood pressure : The normal systolic blood pressure averages about 120 mm Hg; diastolic pressure about 80 mm Hg. Palpable : Perceptible, esp. by touch.
- Infiltration : The deoposition and accumulation of an external substance within a cell, tissue, or organ, such as fat deposition within a damaged liver.
- Convusion : Paroxysms of involuntary muscular contractions and relaxations. Cyanosis : A blue, gray, slate, or dark purple discoloration of the skin or mucous membranes caused by deoxygenated or reduced hemoglobin in the blood. Hypotonia : In physiology, an abnormally low intrinsic resting tension (i.e. low tone in muscle or arteries). Tremor : an involuntary movement of a part or parts of the body resulting from alternate contractions of opposing muscle. Apnea : Temporary cessation of breathing and therefore of the body’s intake of oxygen and release of carbon dioxide. Symphysis : A line of fusion between two bones that are separate in early development, as symphysis of mandible.
- Reminiscent : tending to remind one of something. Eruptive xanthomas : The sudden eruption on the skin of crops of pink papules (firm pea-sized bumps) with a creamy center. They may appear on the hands, feet, arms, legs and buttocks. The papules may be pruritic (itchy).
- A normal level is: Direct (also called conjugated) bilirubin: 0 to 0.3 mg/dL. Total bilirubin: 0.3 to 1.9 mg/dL. Oxidant :The substance that is reduced and that, therefore, oxidizes the other component of an oxidation-reduction system. Polymorphic : Polymorphism, in biology, a discontinuous genetic variation resulting in the occurrence of several different forms or types of individuals among the members of a single species. A discontinuous genetic variation divides the individuals of a population into two or more sharply distinct forms.
- Hemizygous : A chromosome in a diploid organism is hemizygous when only one copy is present. The cell or organism is called a hemizygote. Hemizygosity is also observed when one copy of a gene is deleted, or in the heterogametic sex when a gene is located on a sex chromosome.
- Kernicterus : Kernicterus is a very rare type of brain damage that occurs in a newborn with severe jaundice. It happens when a substance in the blood, called bilirubin, builds up to very high levels and spreads into the brain tissues. This causes permanent brain damage.
- Reticulocyte count : A reticulocyte count is a blood test that measures how fast red blood cells called reticulocytes are made by the bone marrow and released into the blood. Reticulocytes are in the blood for about 2 days before developing into mature red blood cells.
- Indolent : wanting to avoid activity or exertion; lazy. Convulse : suffer violent involuntary contraction of the muscles, producing contortion of the body or limbs. Accentuated make more noticeable or prominent.
- Benign : (of a disease) not harmful in effect.
- Cardinal : of the greatest importance; fundamental.
- Ataxia : defective muscular coordination, esp that manifested when voluntary muscular movements are attempted.
