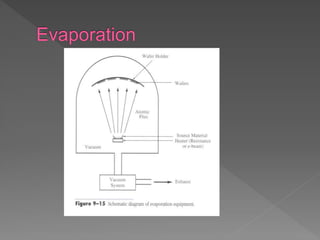
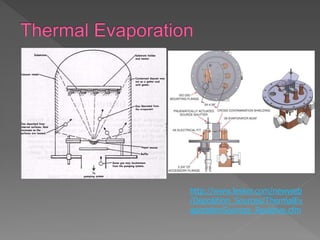
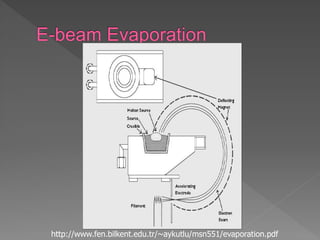
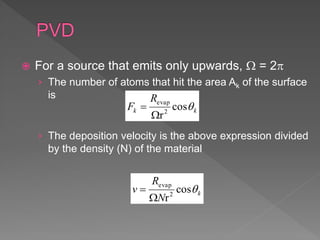
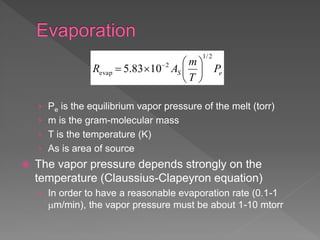
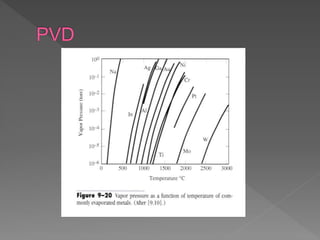
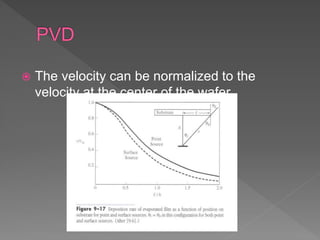
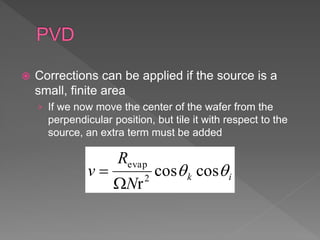
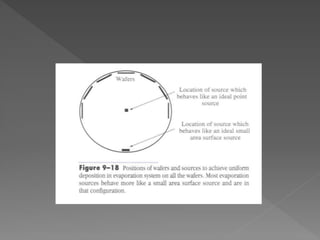
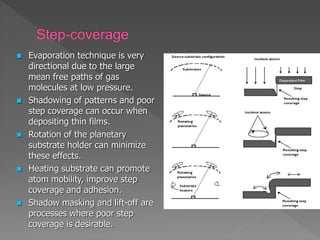
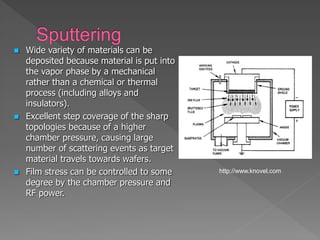
Physical vapor deposition (PVD) involves evaporating or sputtering material from a source to deposit thin films on a substrate in a vacuum chamber. In evaporation, a thermal source heats material which travels in straight lines to the substrate. Sputtering uses plasma to bombard a target, ejecting atoms which deposit with better step coverage. Both techniques can deposit a wide range of materials but sputtering provides better step coverage and evaporation risks contamination.