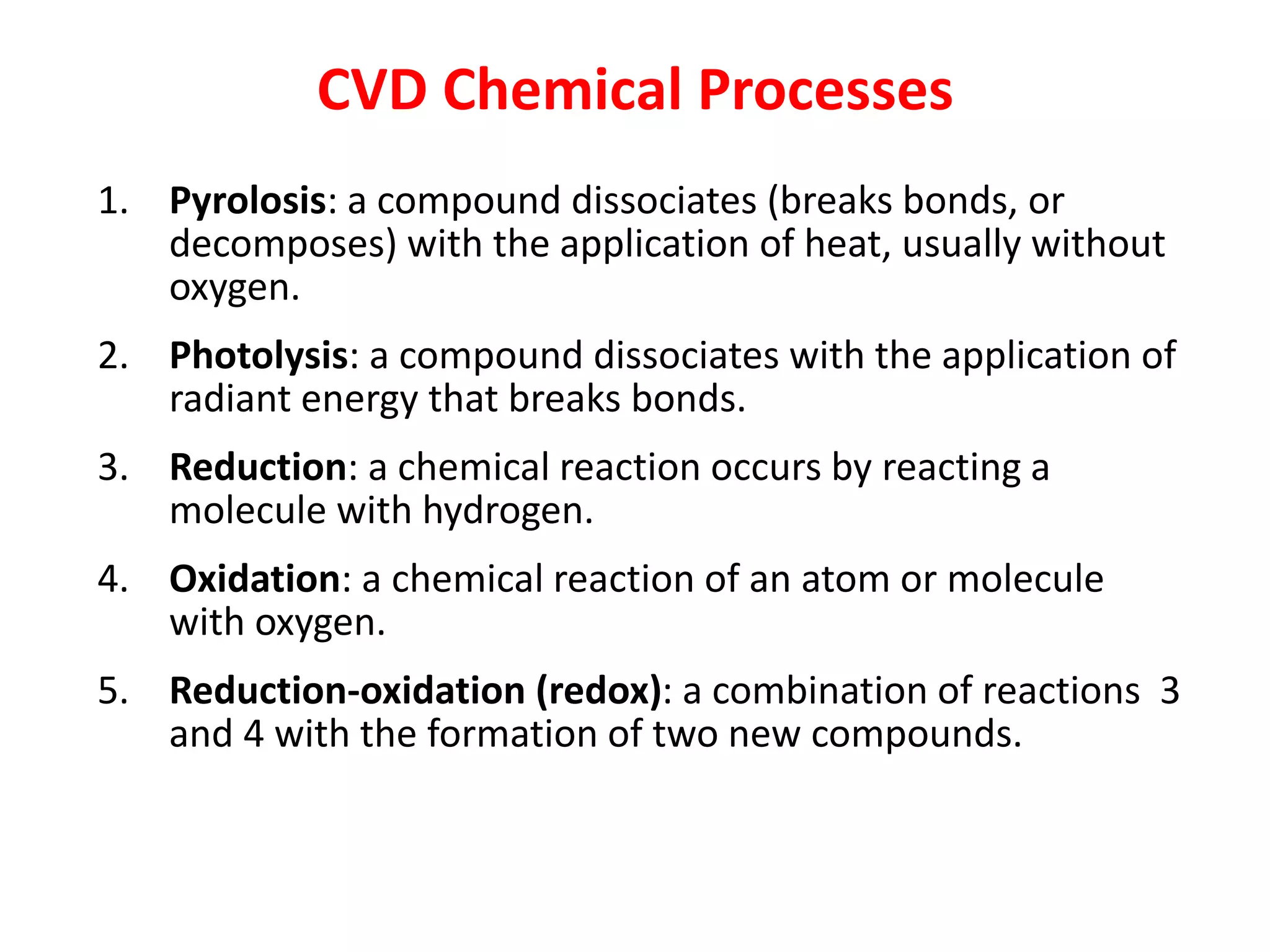
The document discusses thin film deposition techniques used in semiconductor manufacturing. It covers physical vapor deposition (PVD) methods like evaporation and sputtering as well as chemical vapor deposition (CVD). Evaporation involves heating a source material to produce a vapor that deposits on wafers in a vacuum chamber. Sputtering uses argon plasma to eject atoms from a target material that then deposit on wafers. CVD techniques like APCVD and LPCVD use chemical reactions or decomposition of precursor gases to deposit thin films. The document discusses parameters, characteristics and challenges of thin film deposition methods.