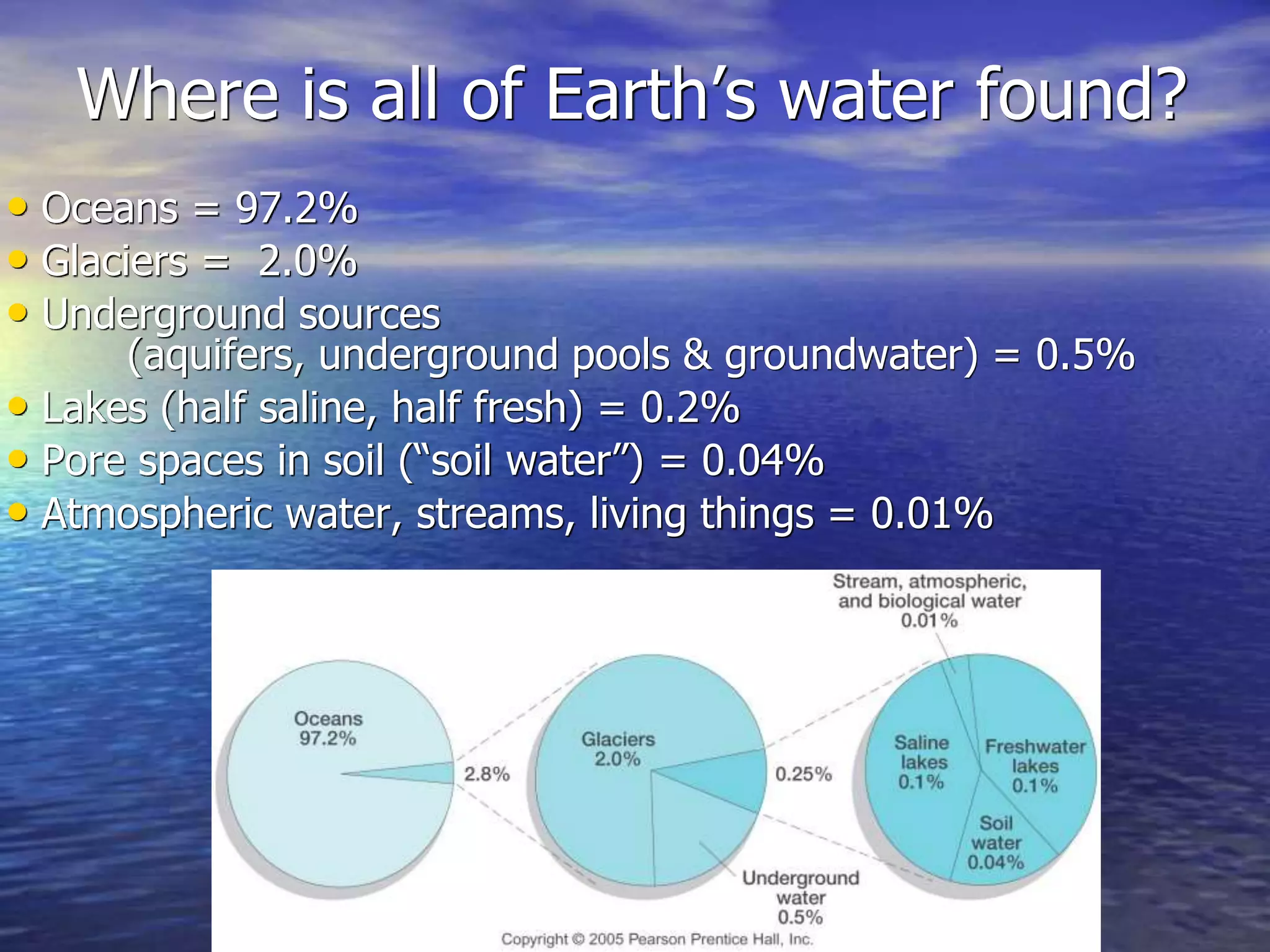
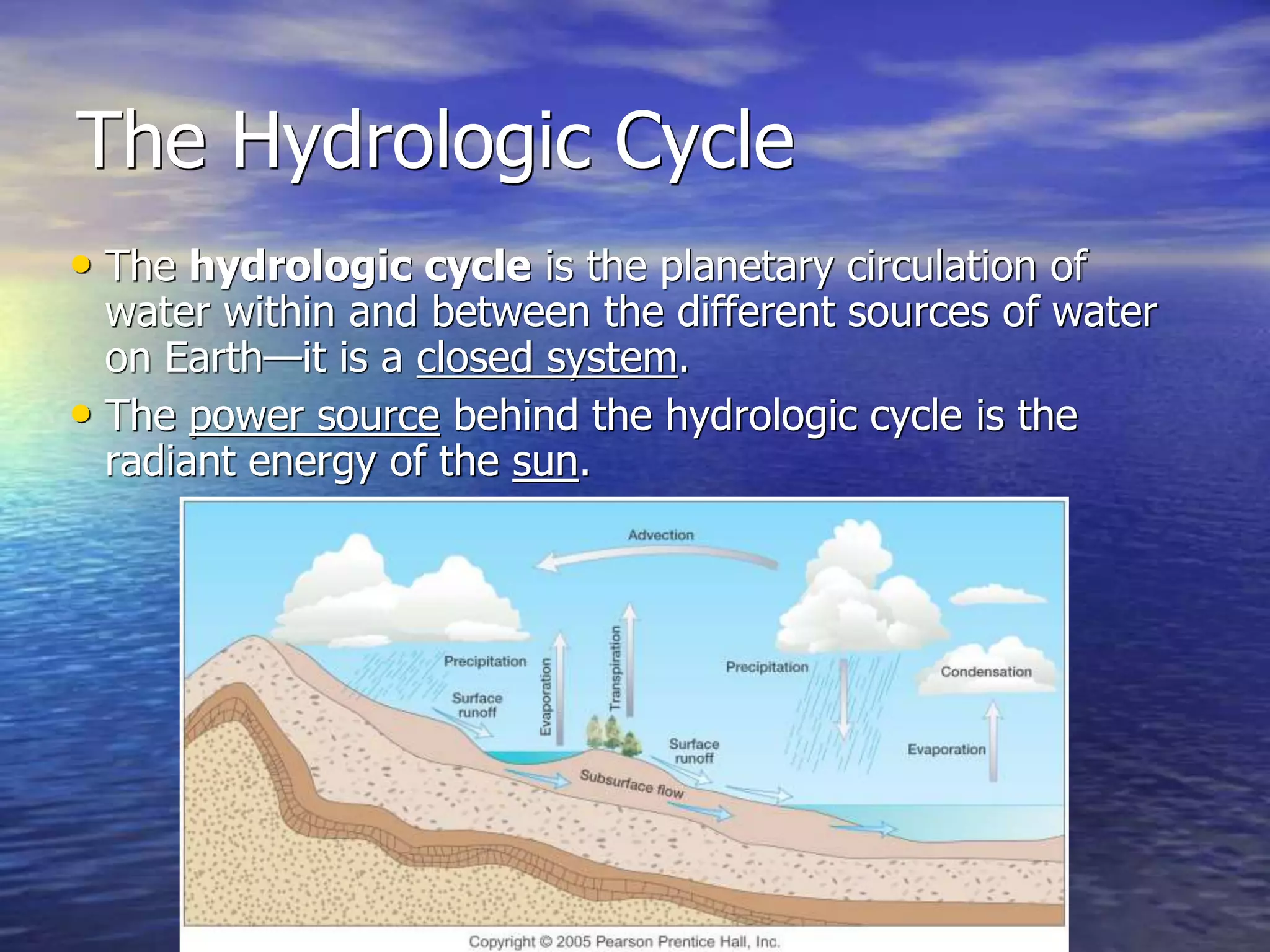
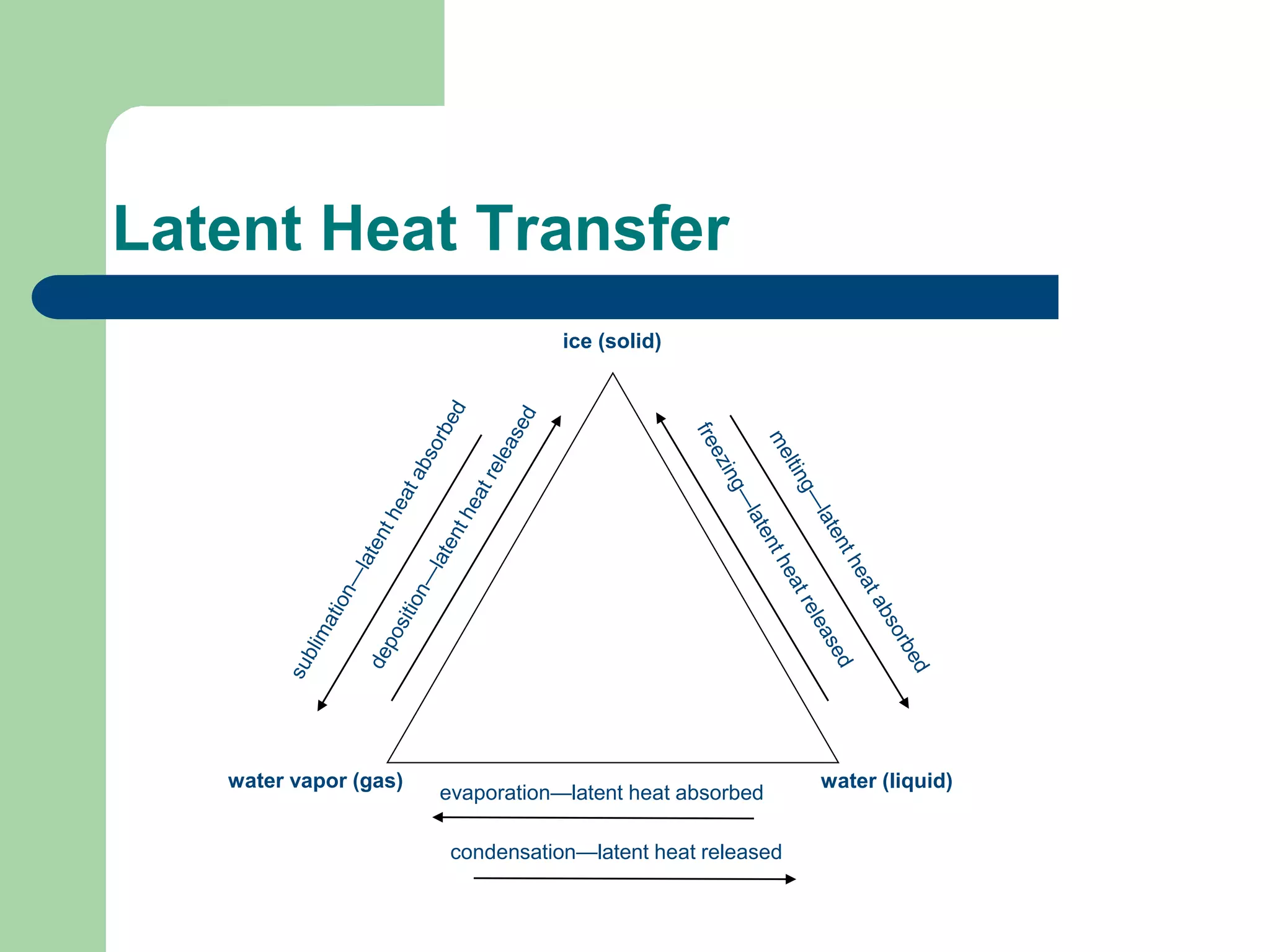
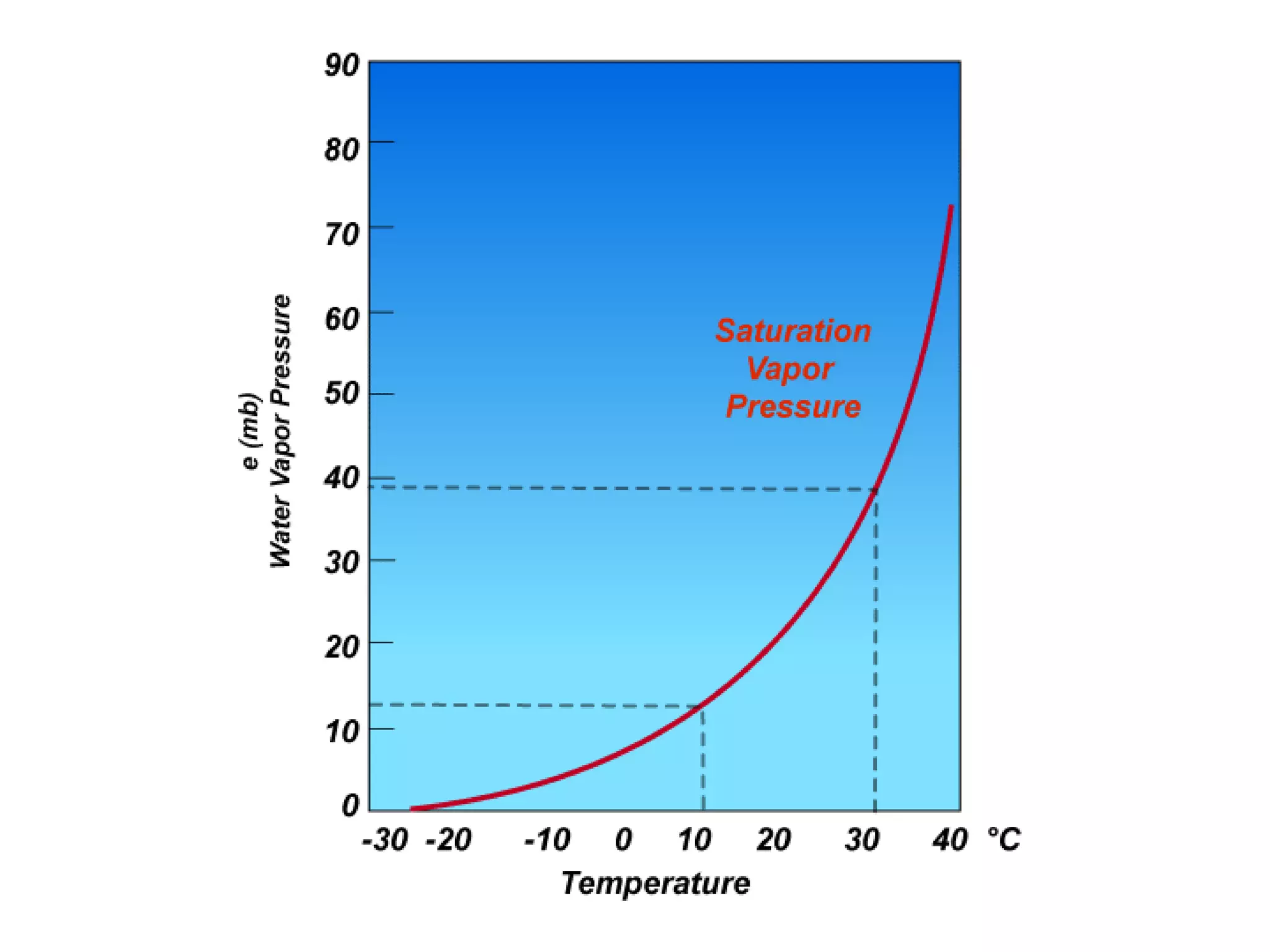
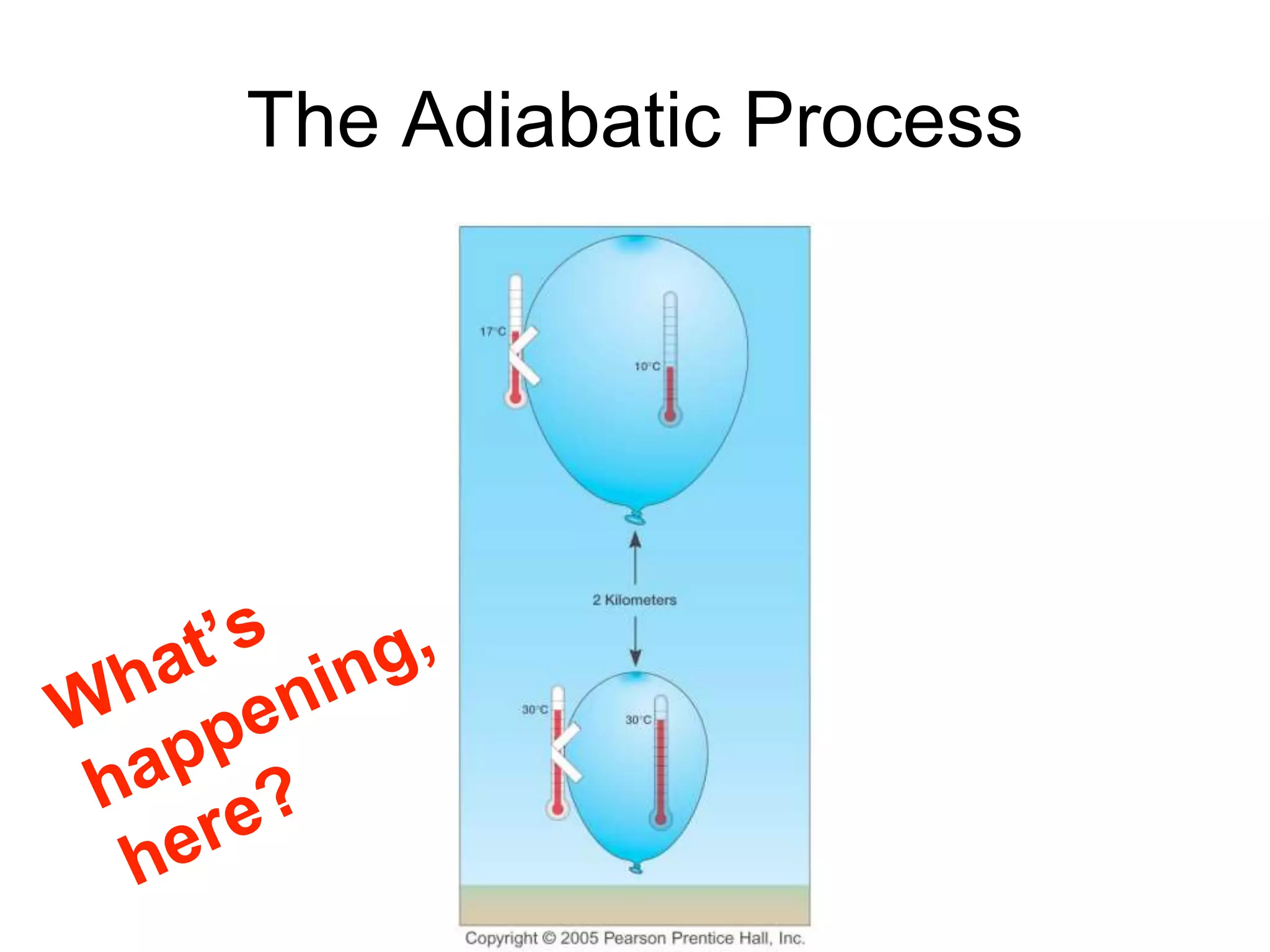
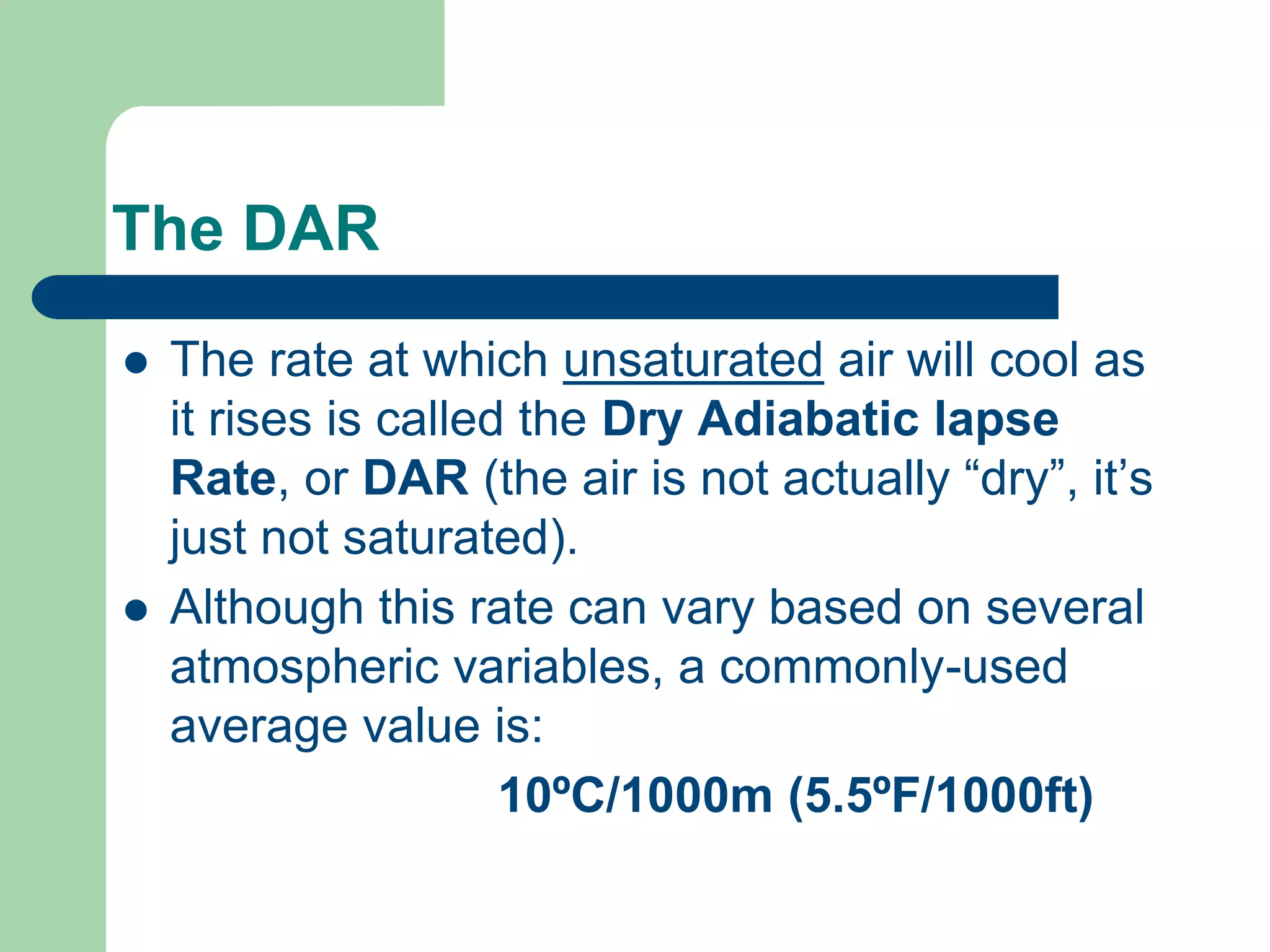
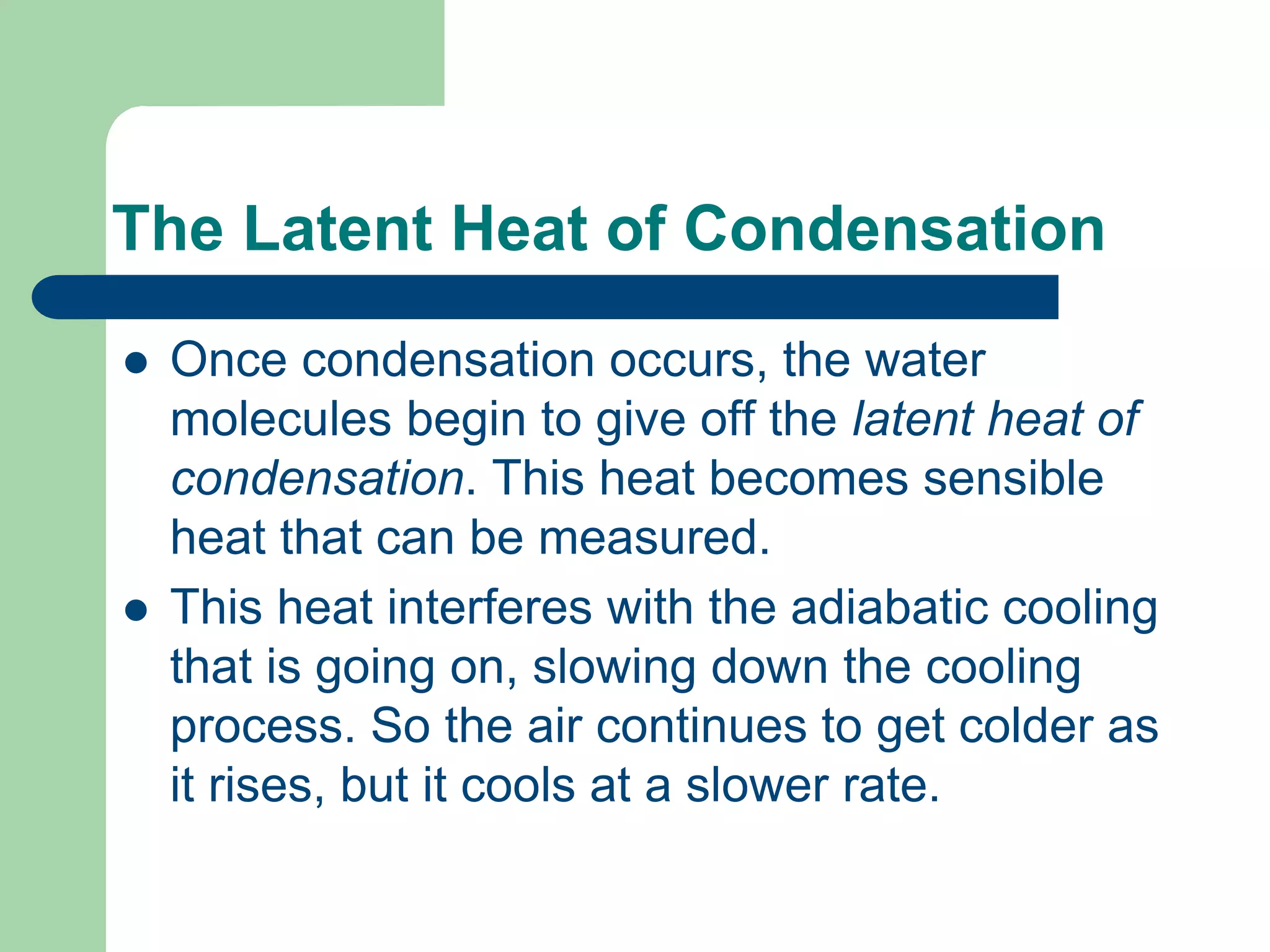
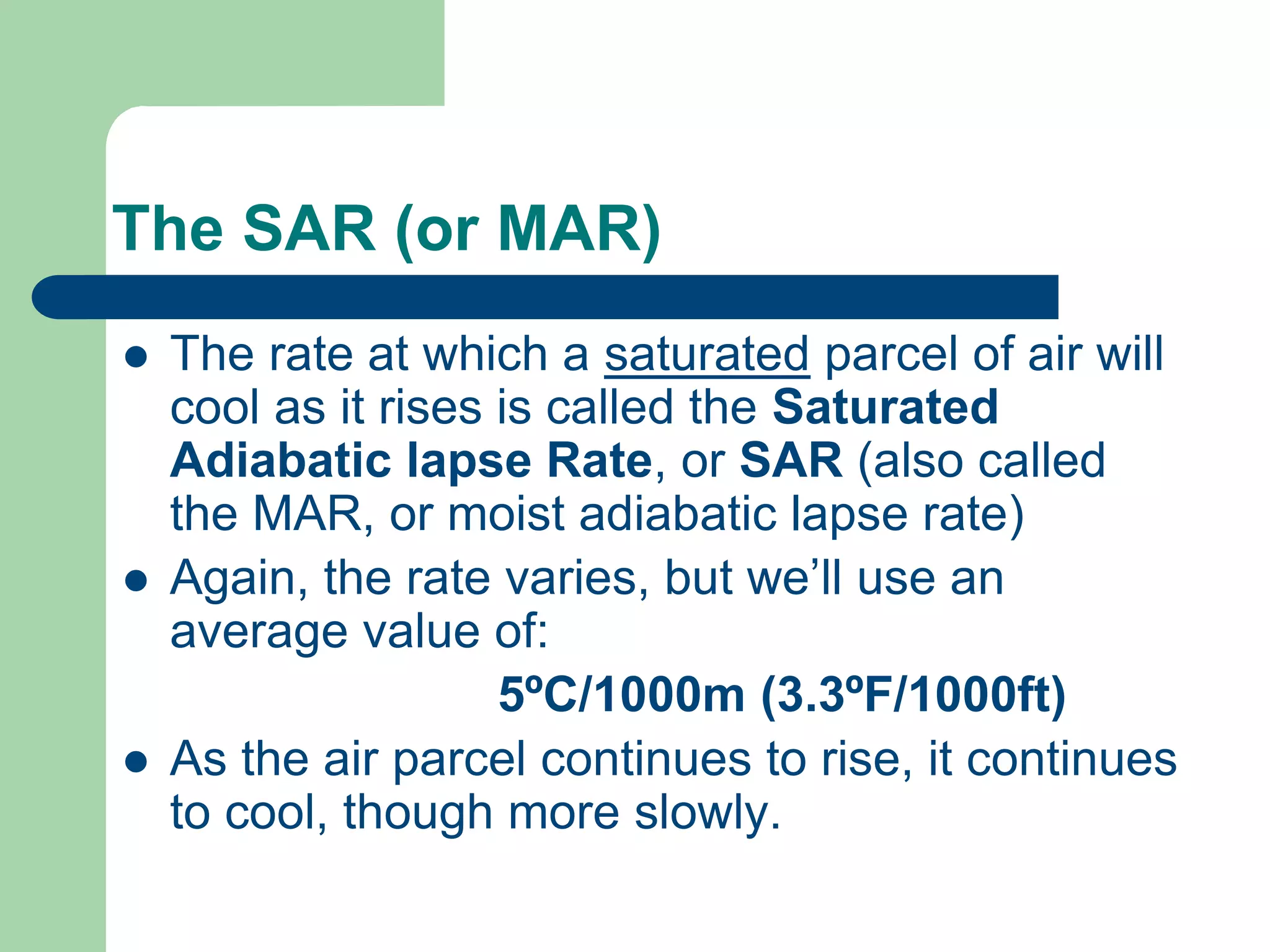
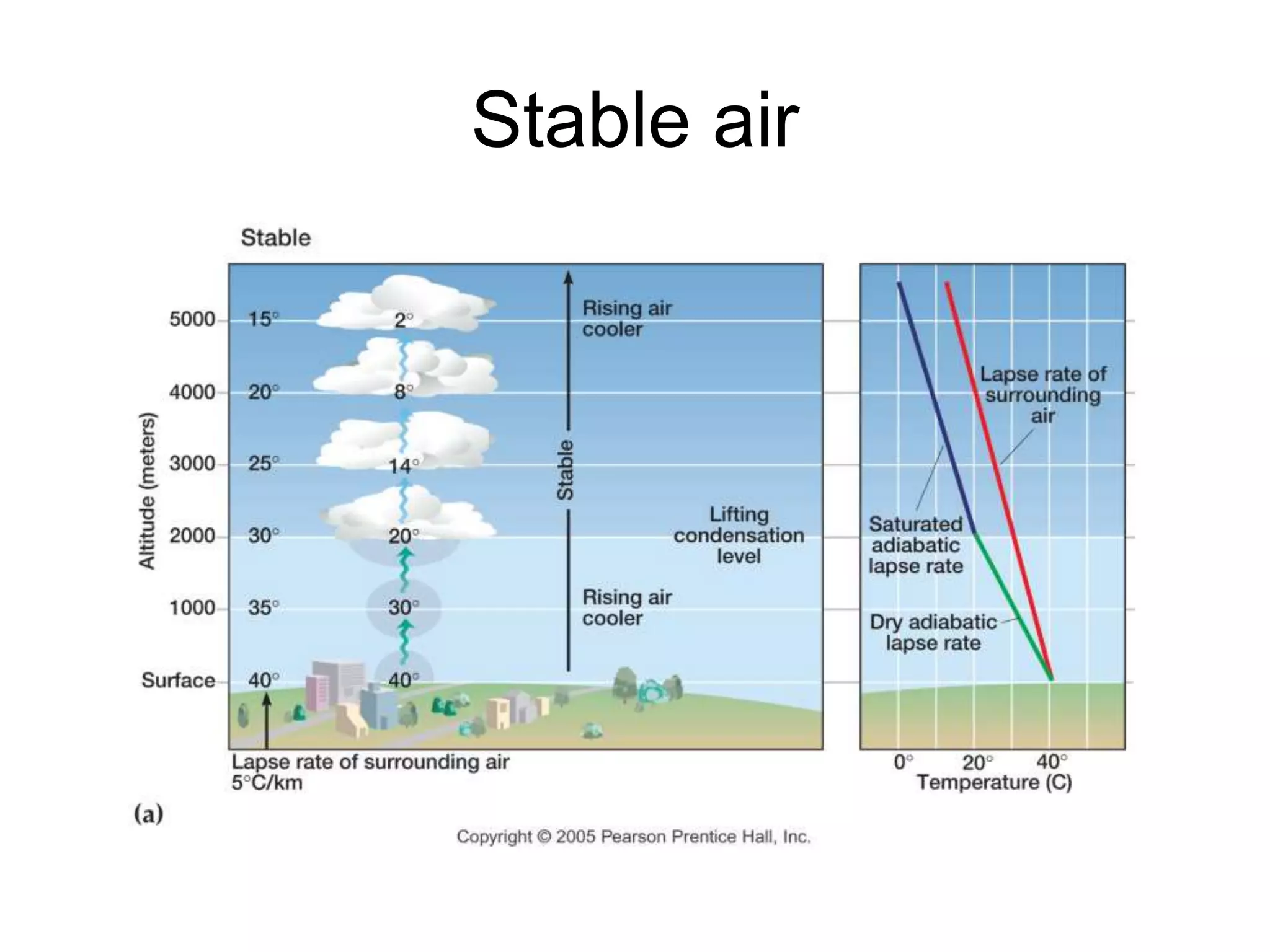
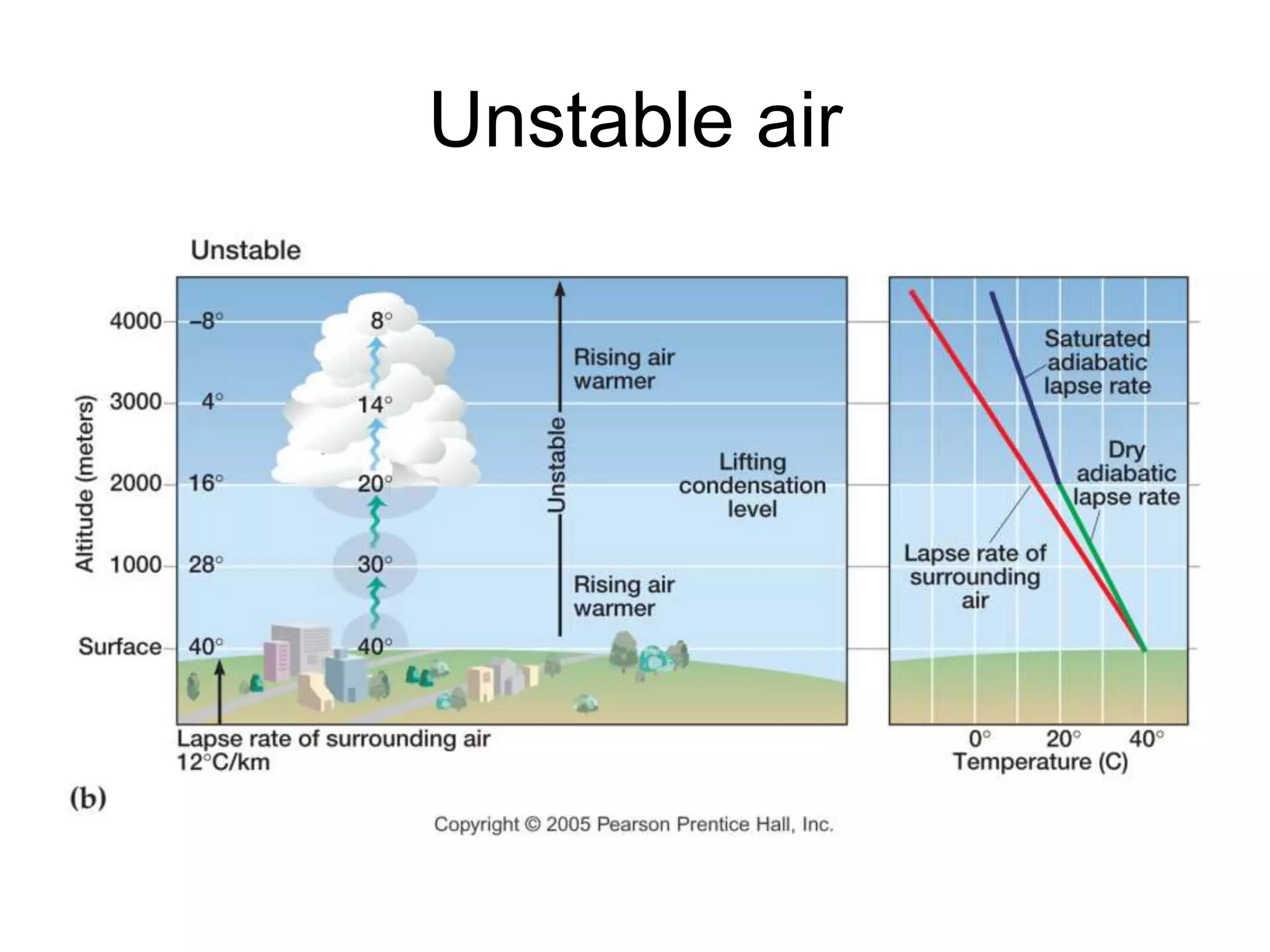
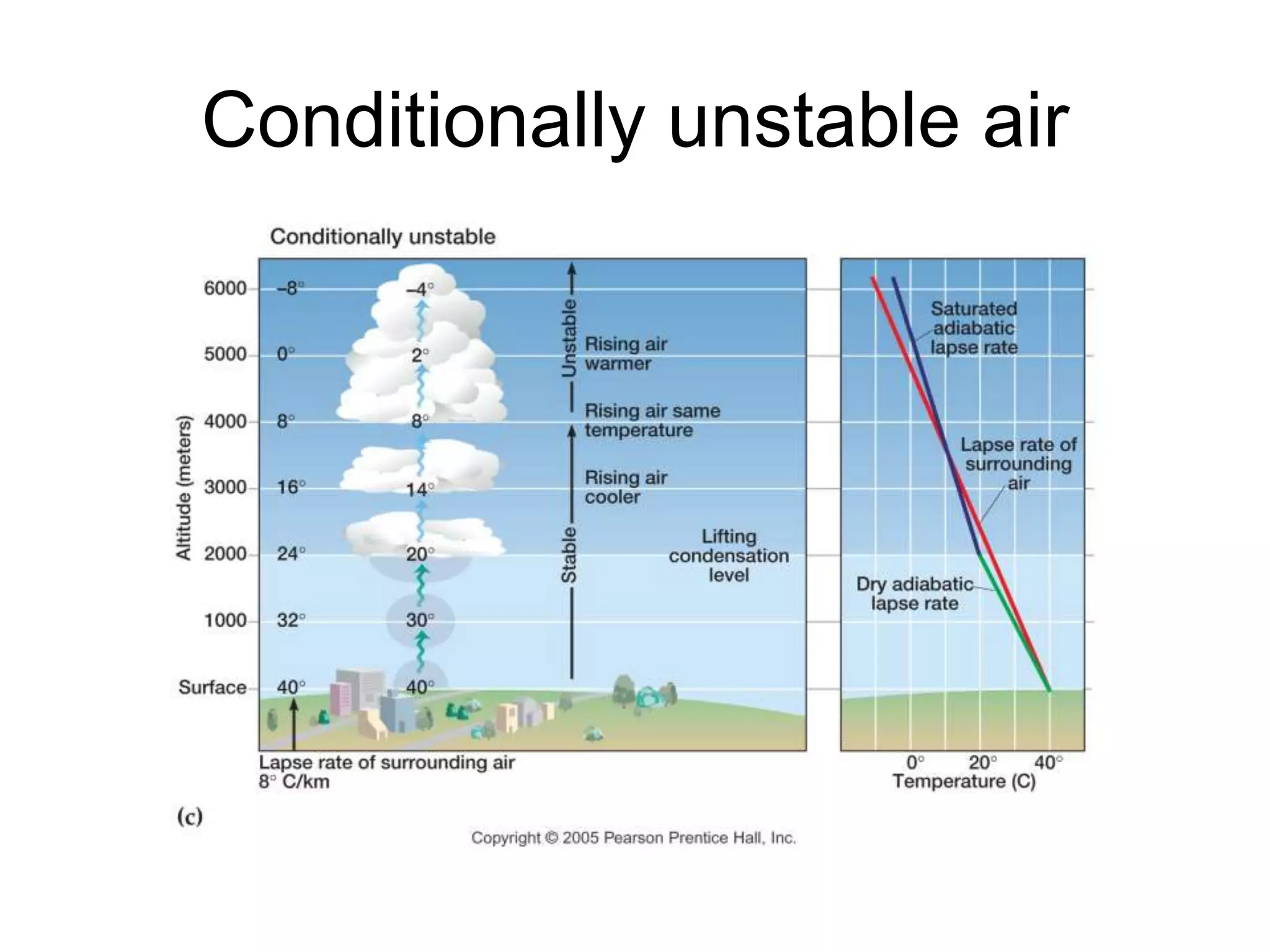
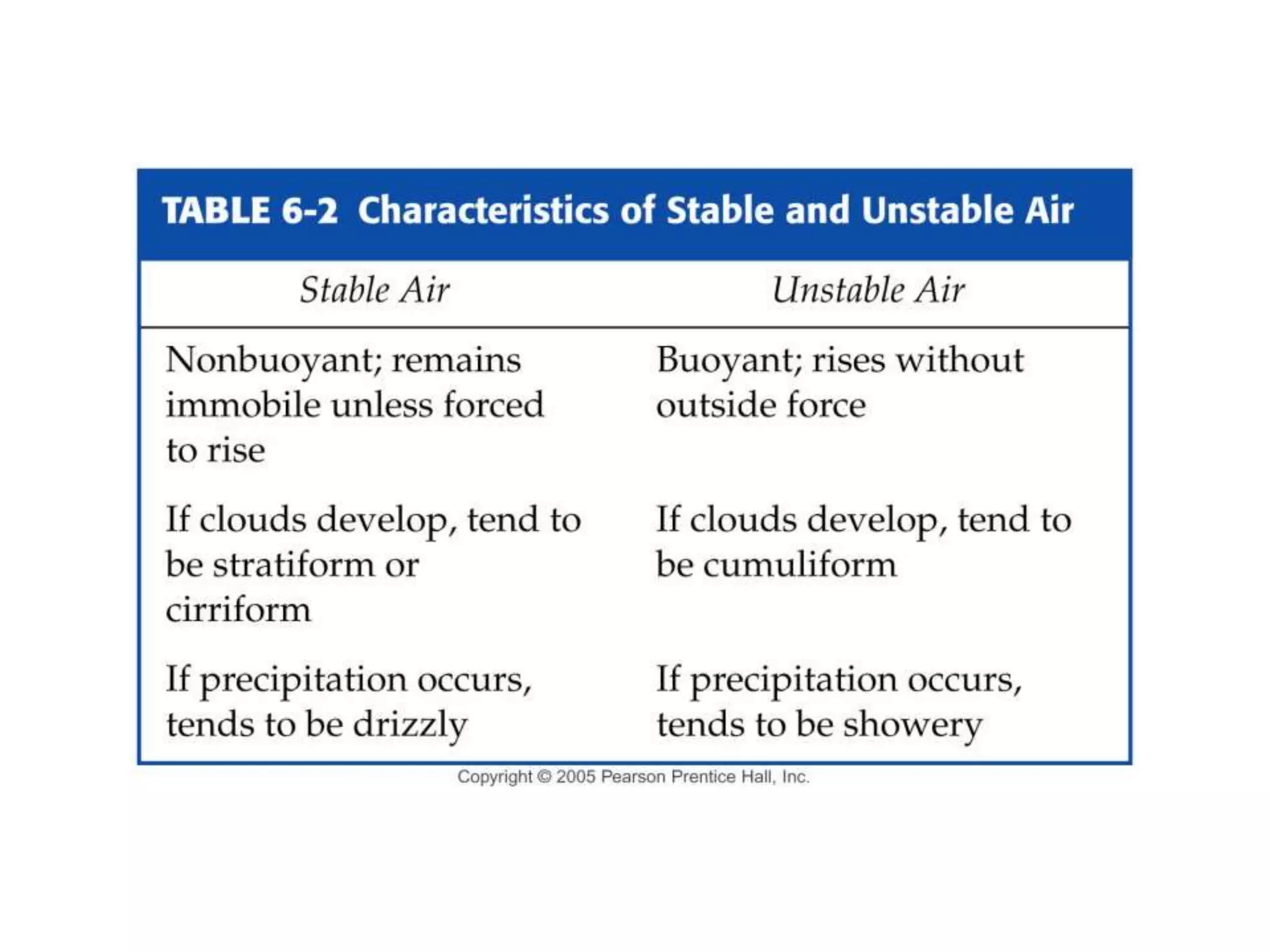
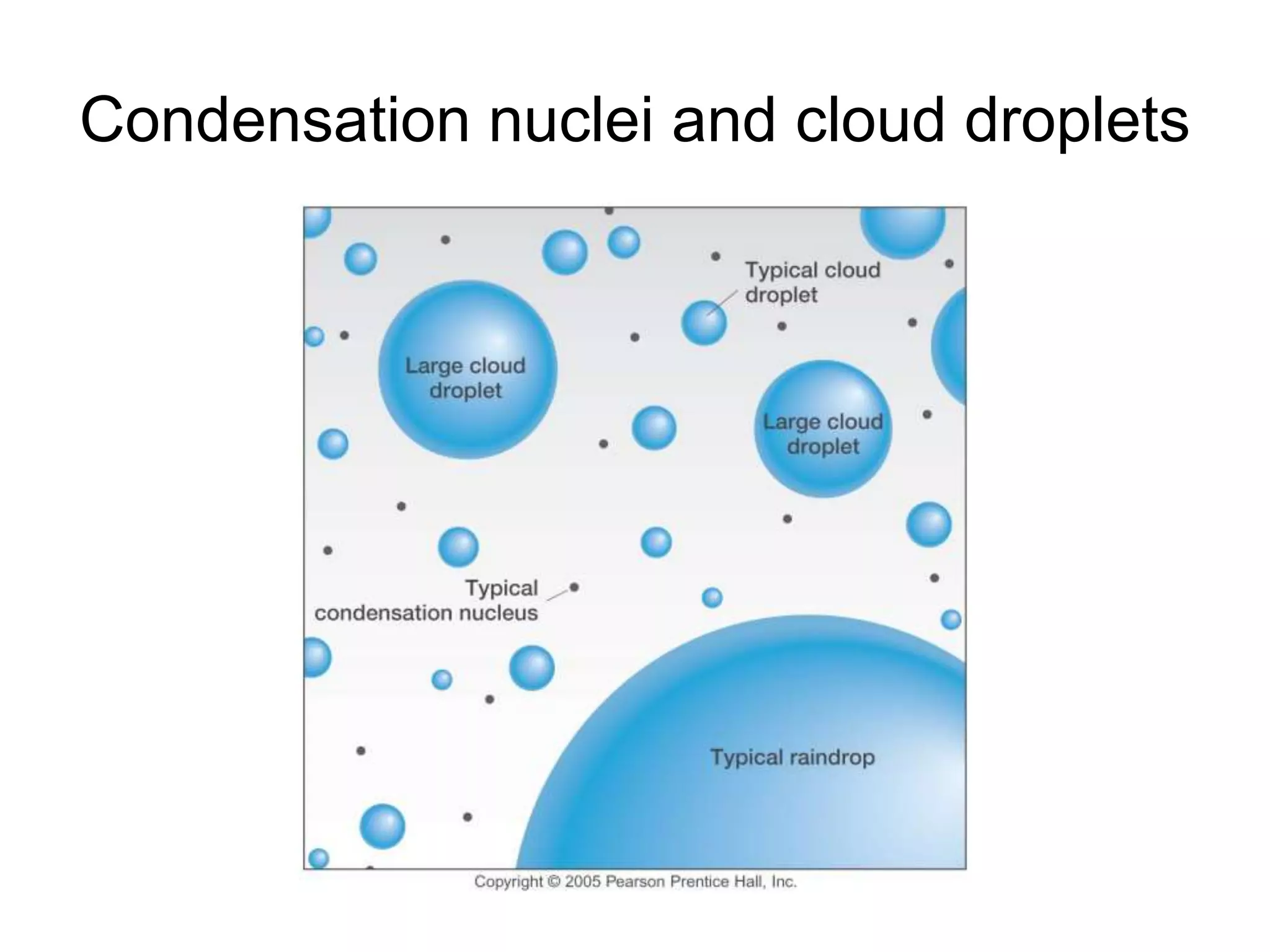
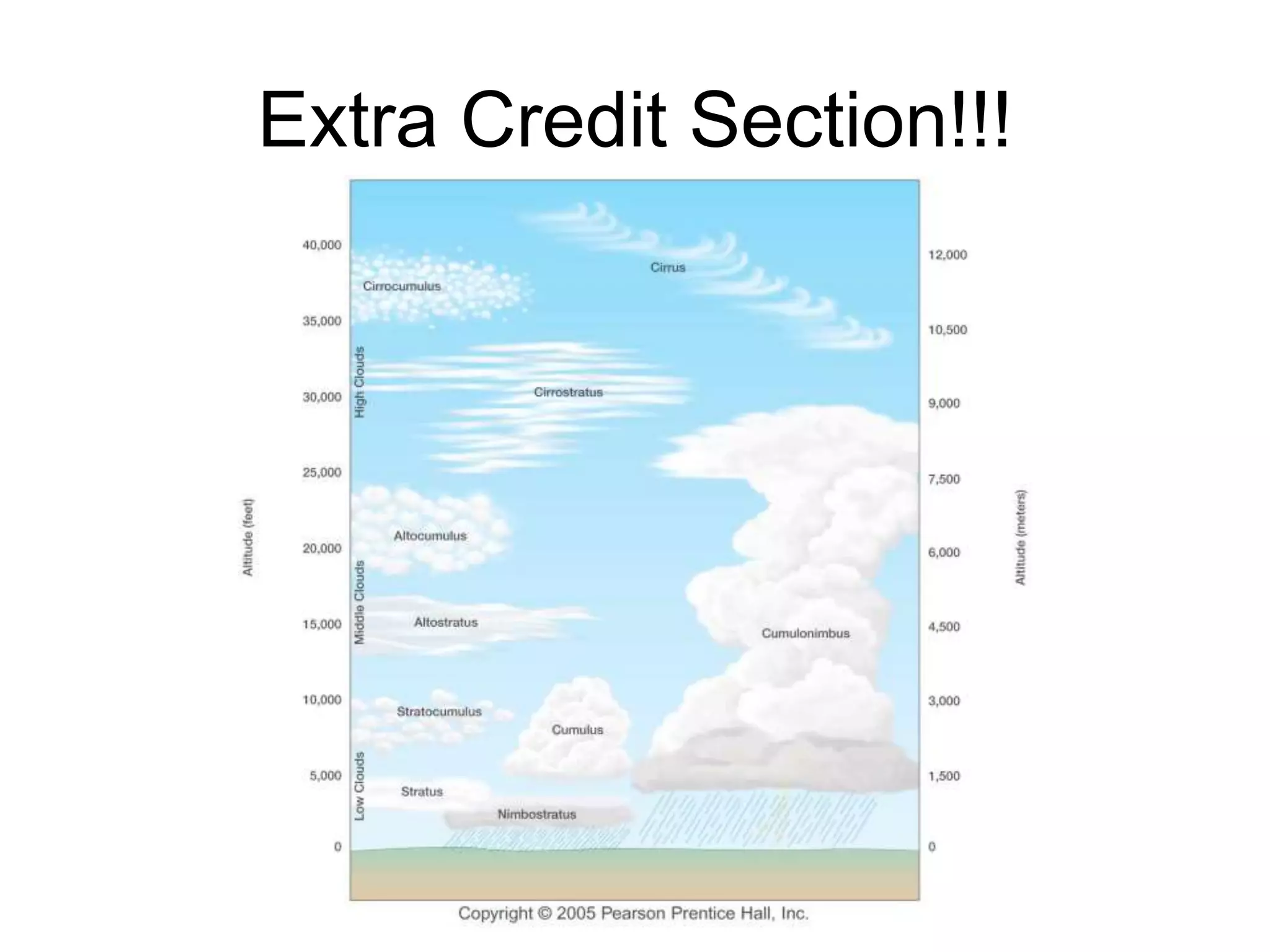
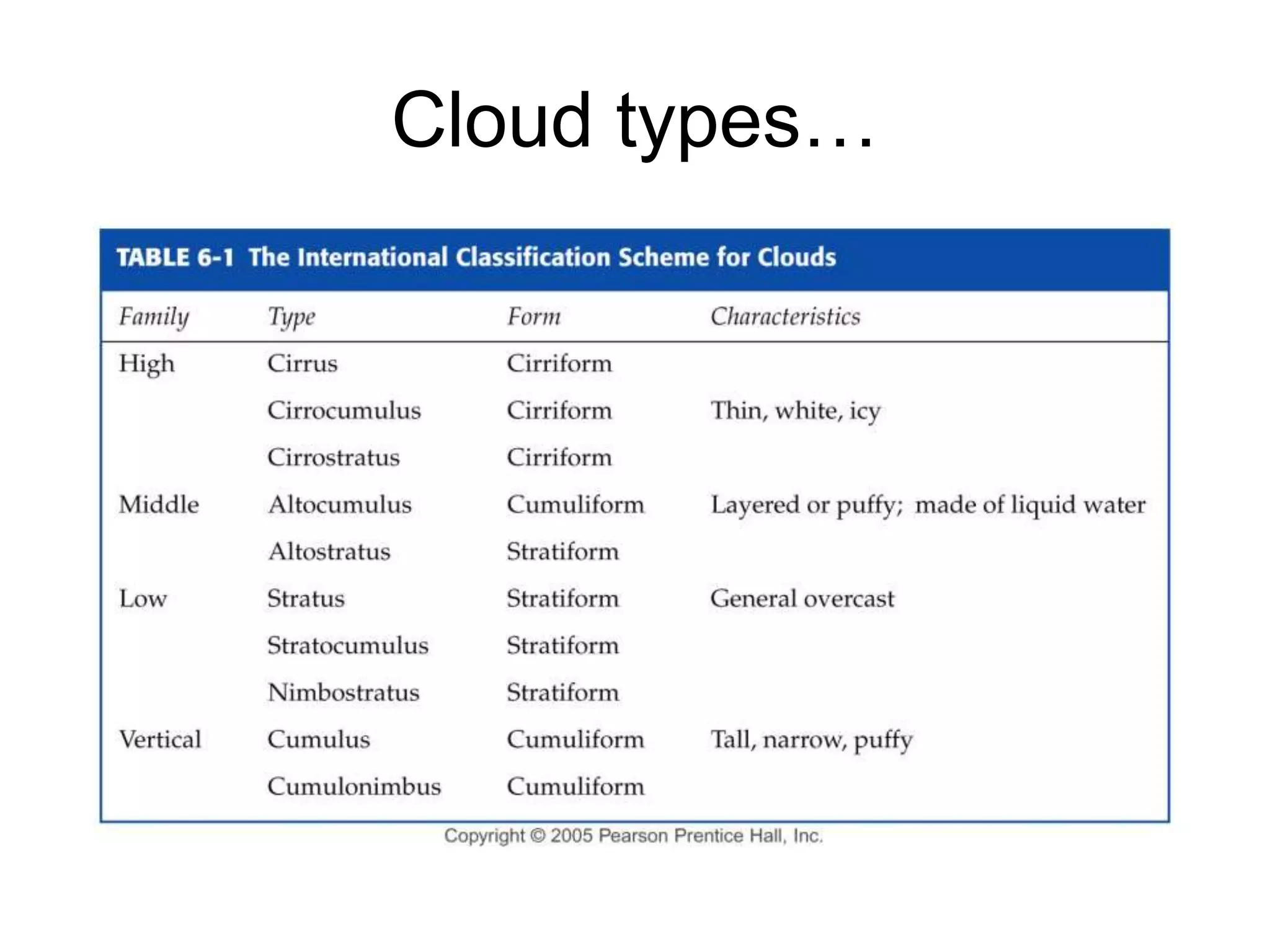
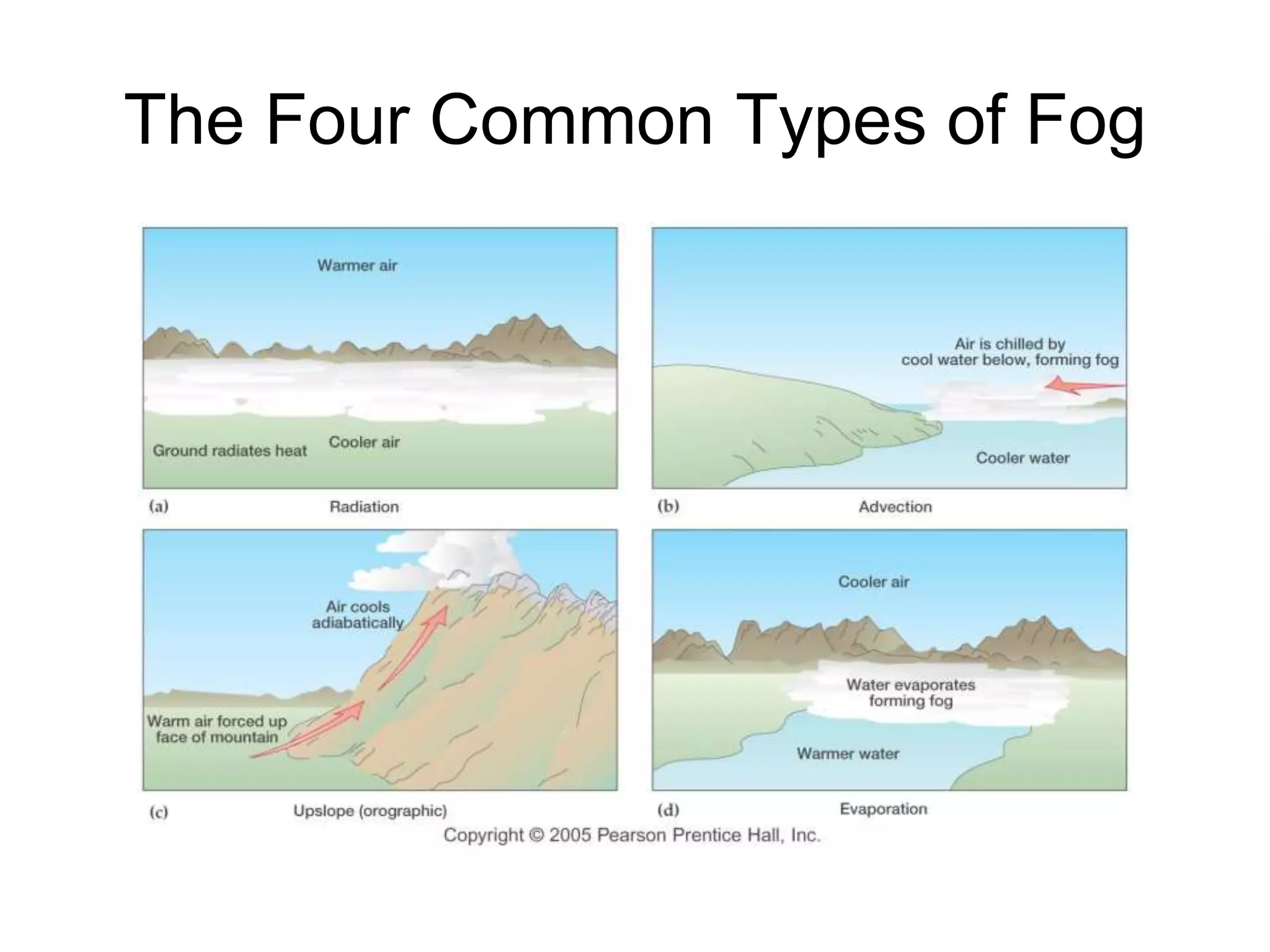
Chapter 5 of physical geography covers clouds and precipitation, detailing the hydrologic cycle and Earth's water distribution, with oceans containing 97.2% of the total water. It explains concepts such as residence time, relative humidity, and the adiabatic process that influences air temperature and cloud formation. The chapter also discusses conditions leading to saturation, condensation, and various types of fog and clouds.