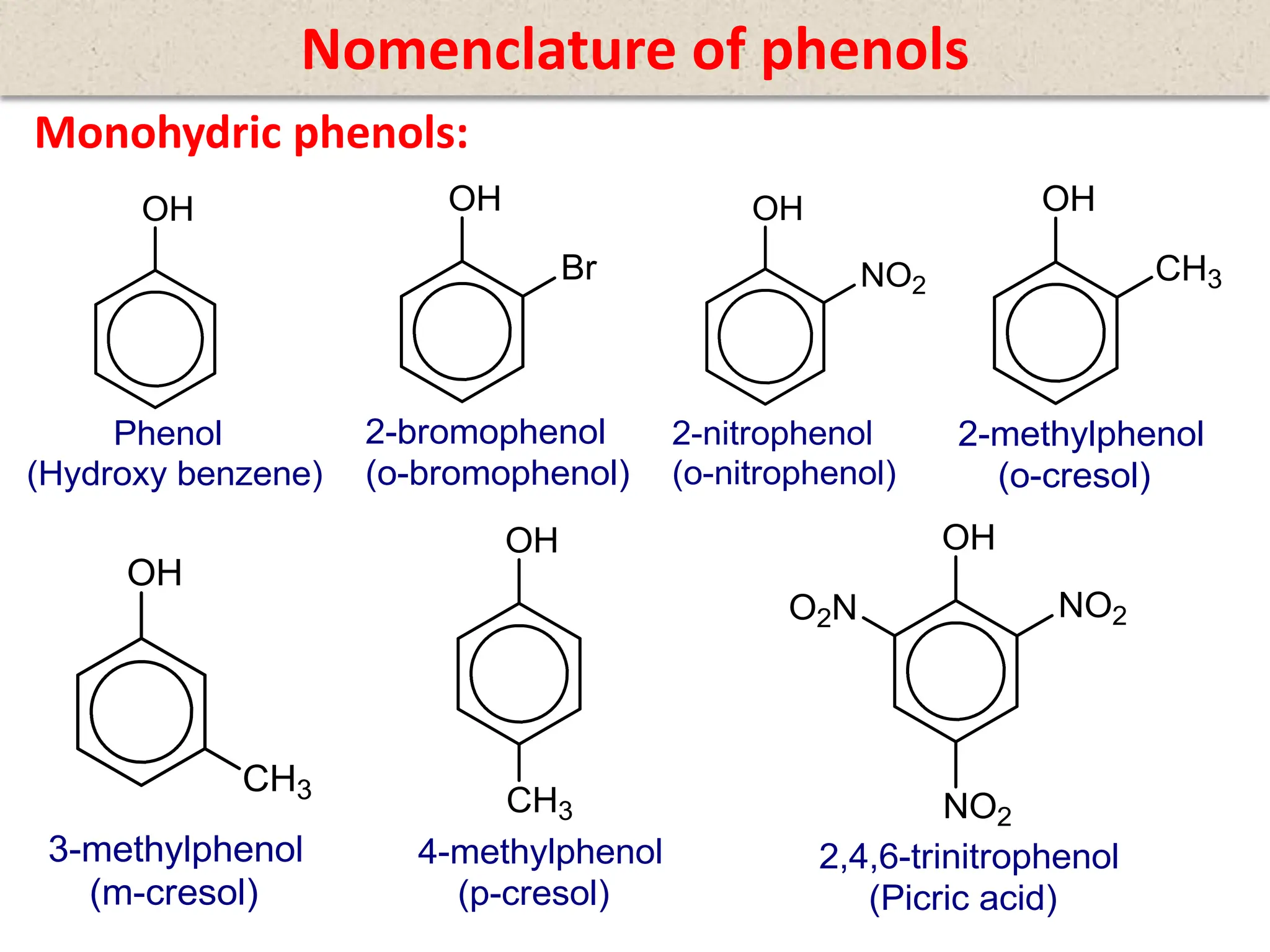
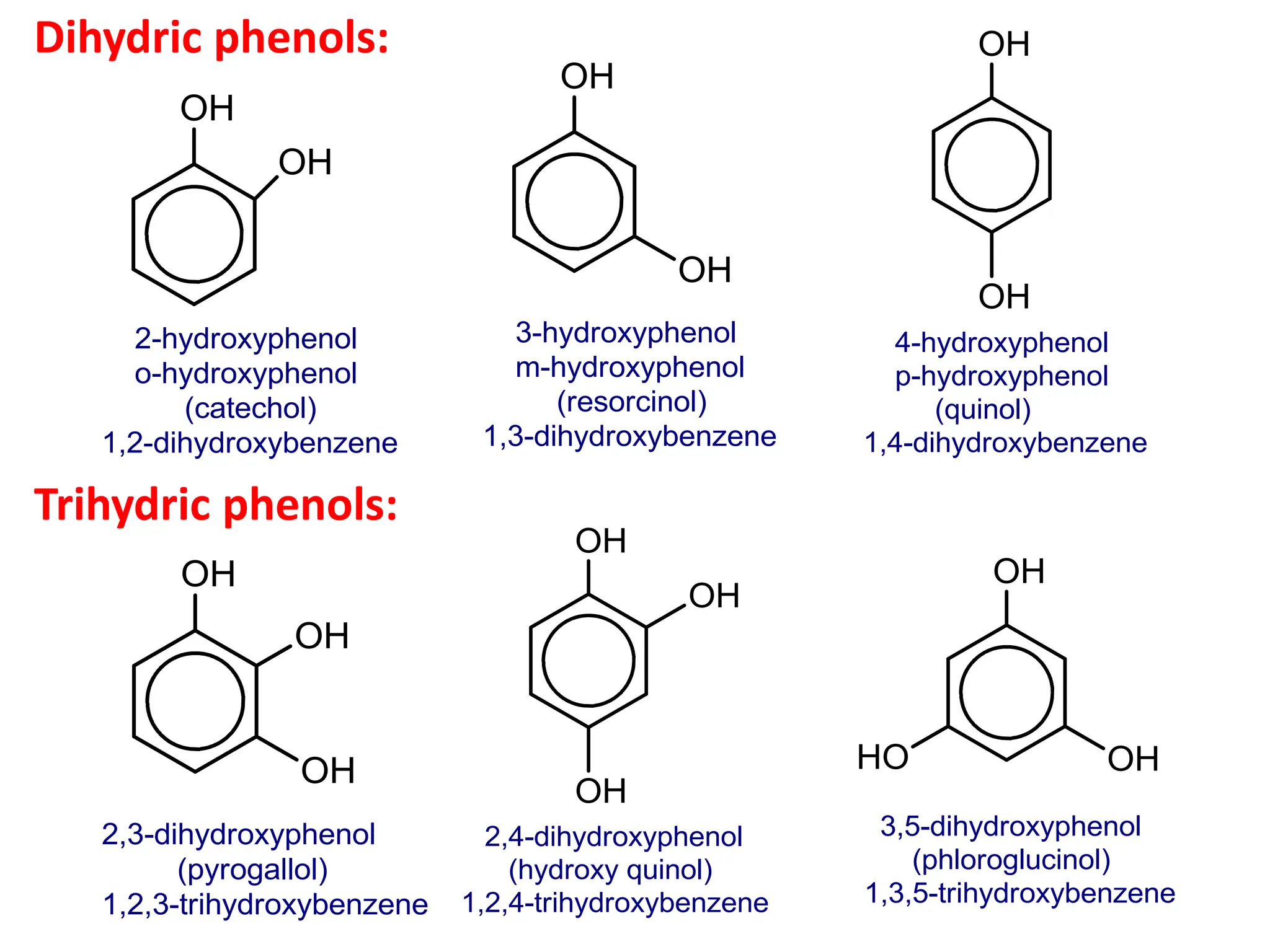
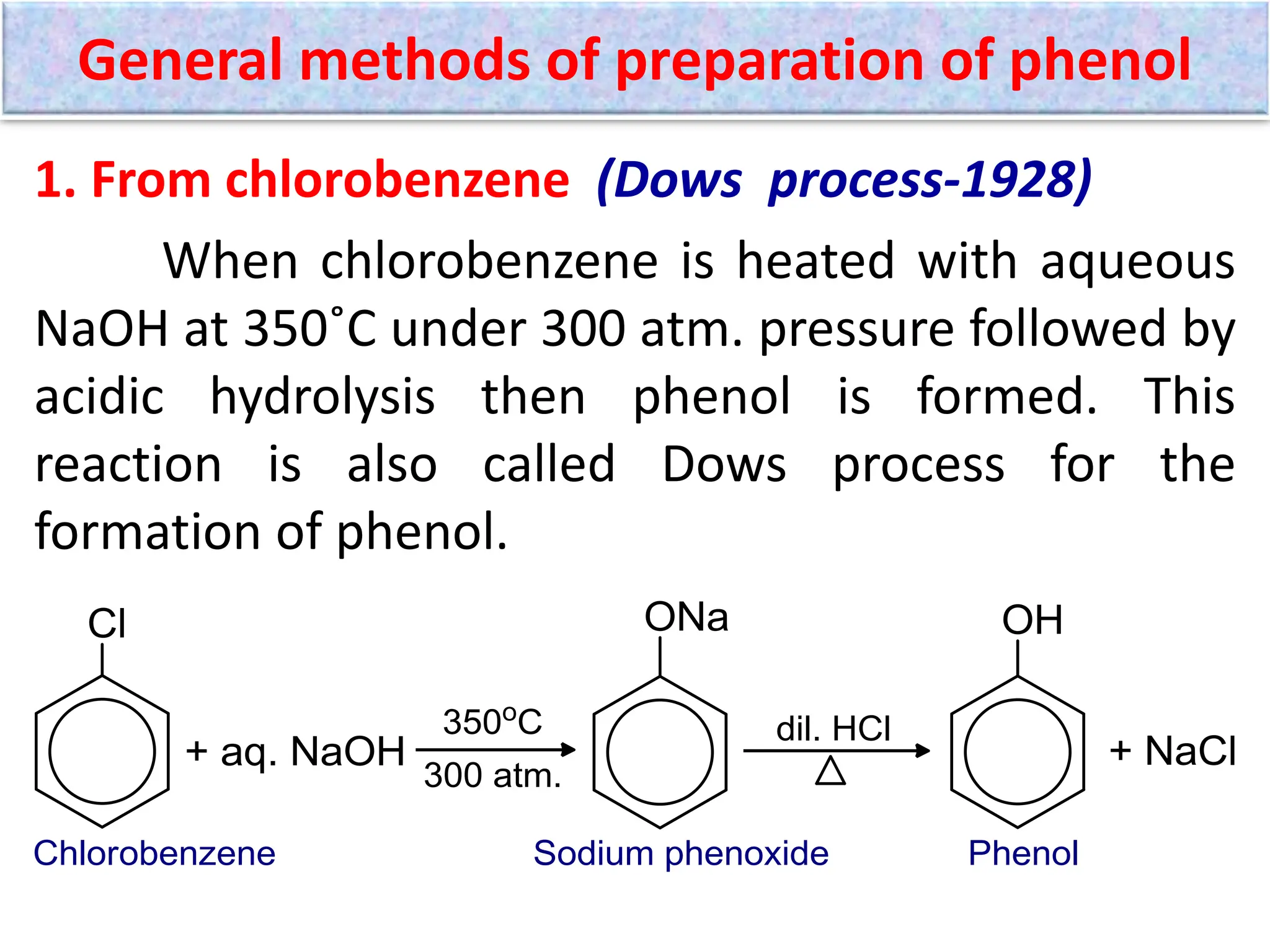
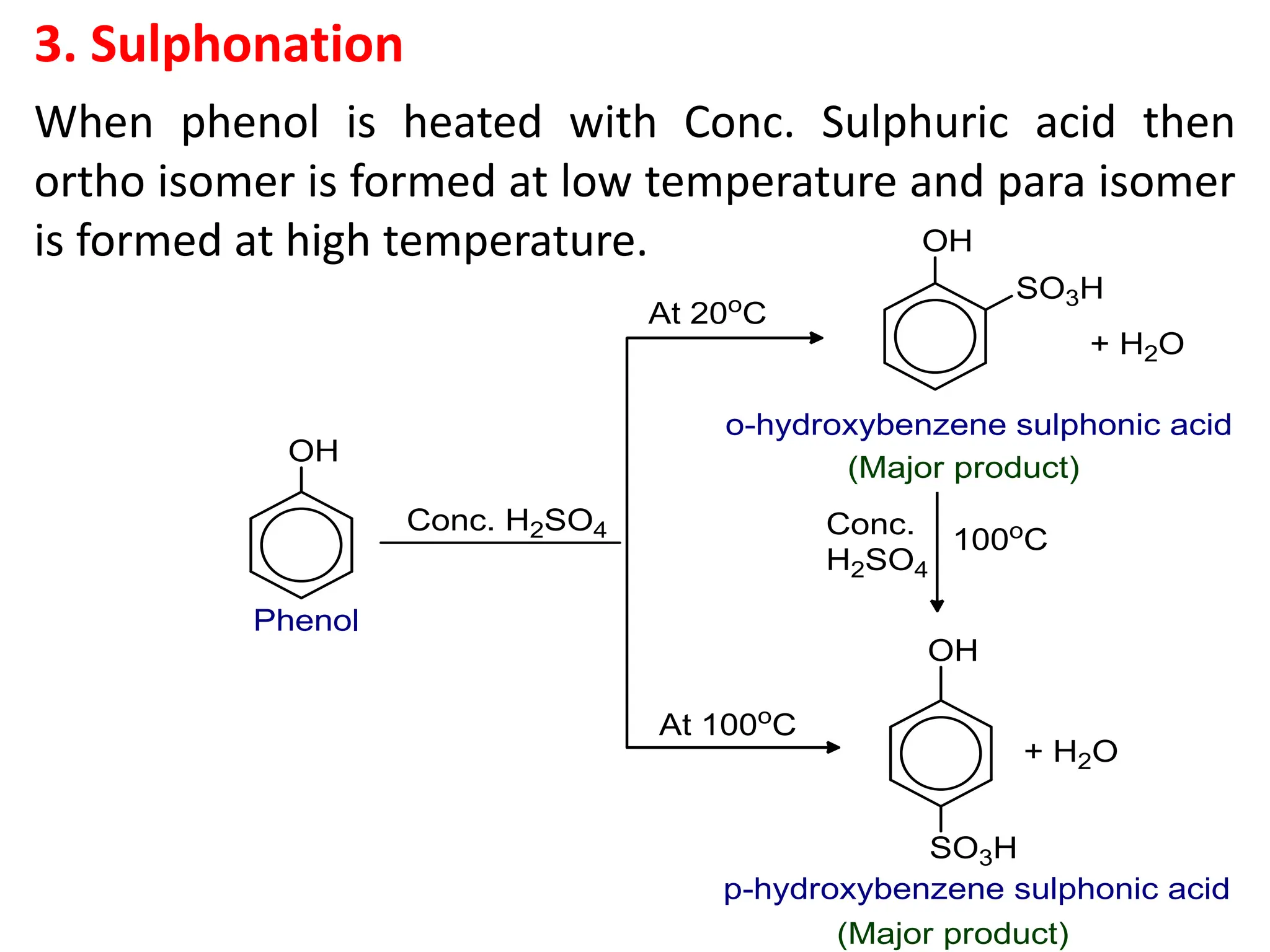
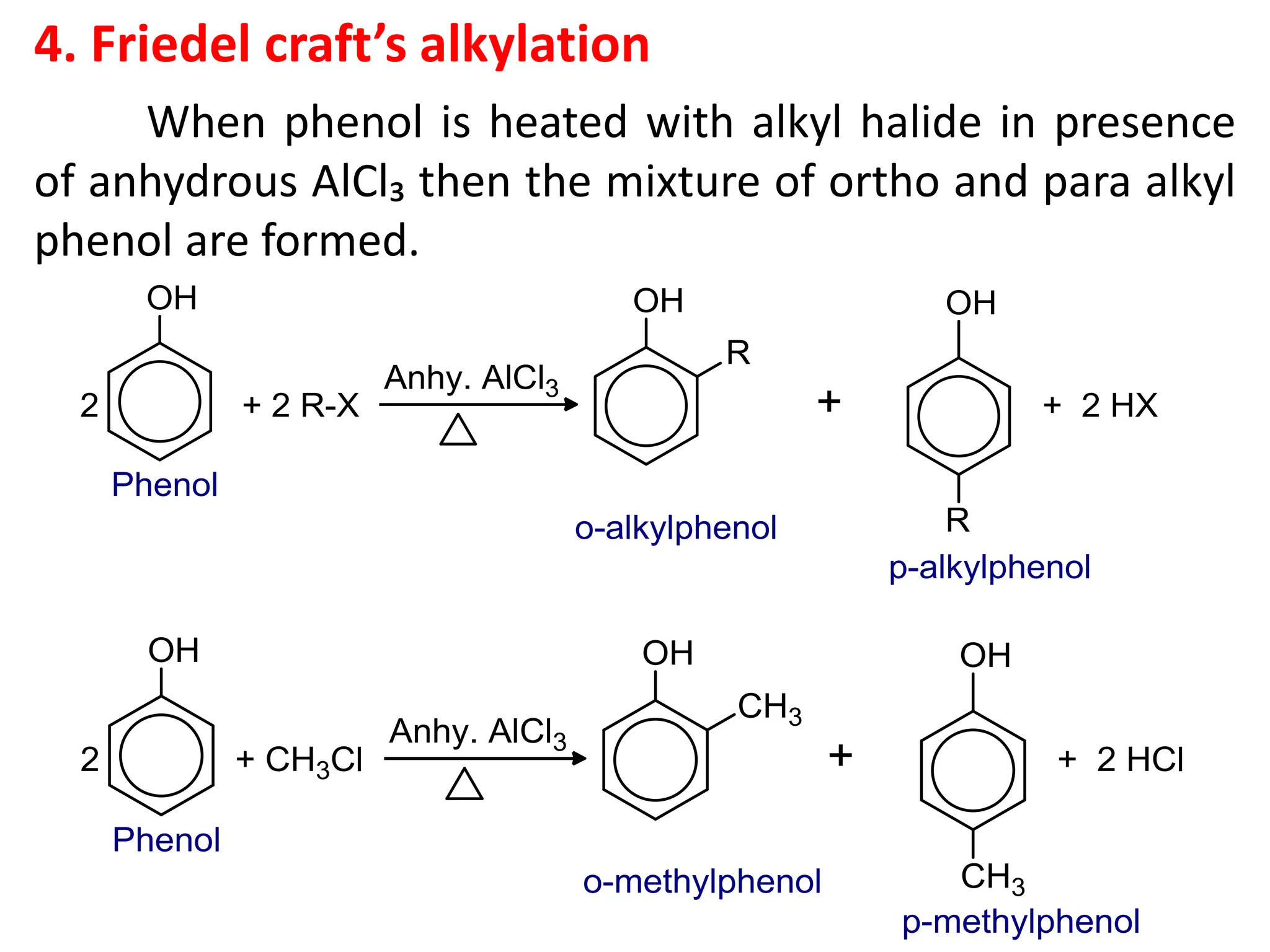
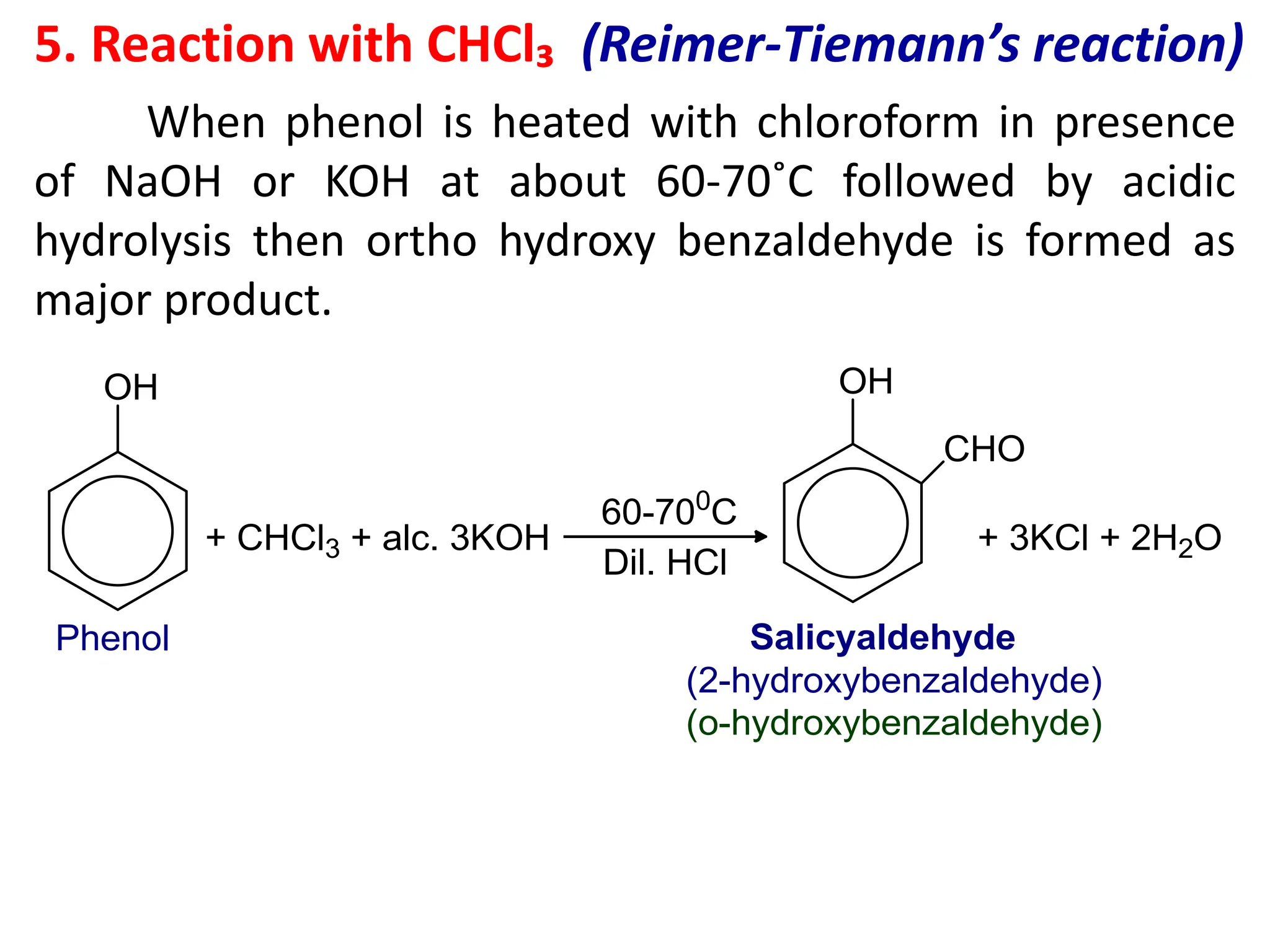
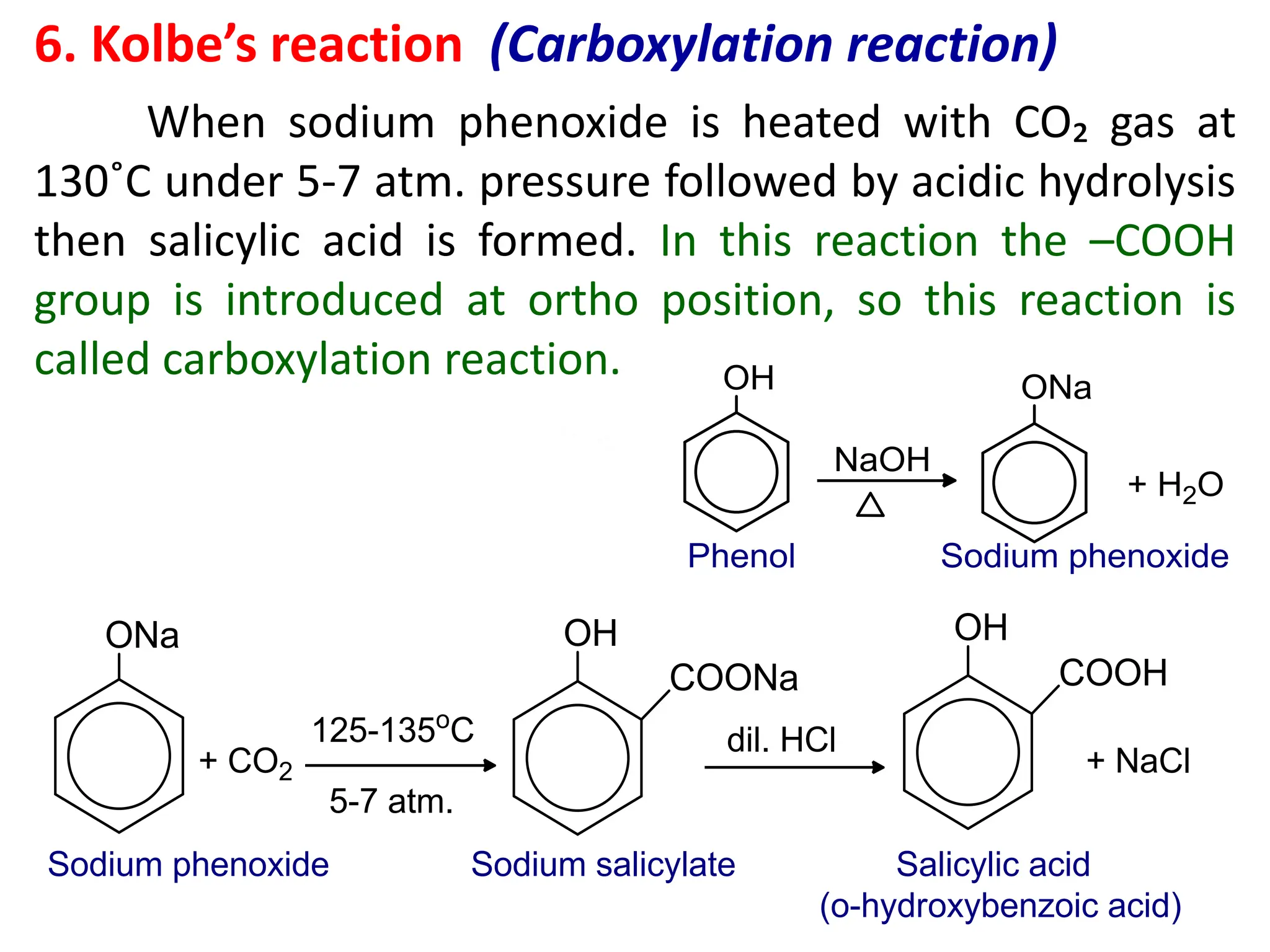
The document provides a detailed overview of phenols, including their definition, nomenclature, preparation methods, physical and chemical properties, tests, and applications. Key topics include the classification of phenols (monohydric, dihydric, and trihydric), acidity comparison with carboxylic acids, and reactions such as electrophilic substitution, oxidation, and synthesis of various derivatives. It also includes examination questions related to phenols and their chemical behavior.

![Phenols
Introduction
The hydroxy derivative of arenes (Aromatic
hydrocarbon) in which the –OH group is directly
attached to carbon atom of aromatic ring are called
phenols. They are formed by replacing one or more
hydrogen atom by –OH group in benzene ring.
[The –OH group is bonded to
carbon atom of side chain of
aromatic ring are called
aromatic alcohol]](https://image.slidesharecdn.com/4-241130161016-baa03818/75/4-Phenols-pdf-which-explain-about-the-general-methods-of-preparation-of-phenols-physical-and-chemical-properties-and-it-s-uses-3-2048.jpg)

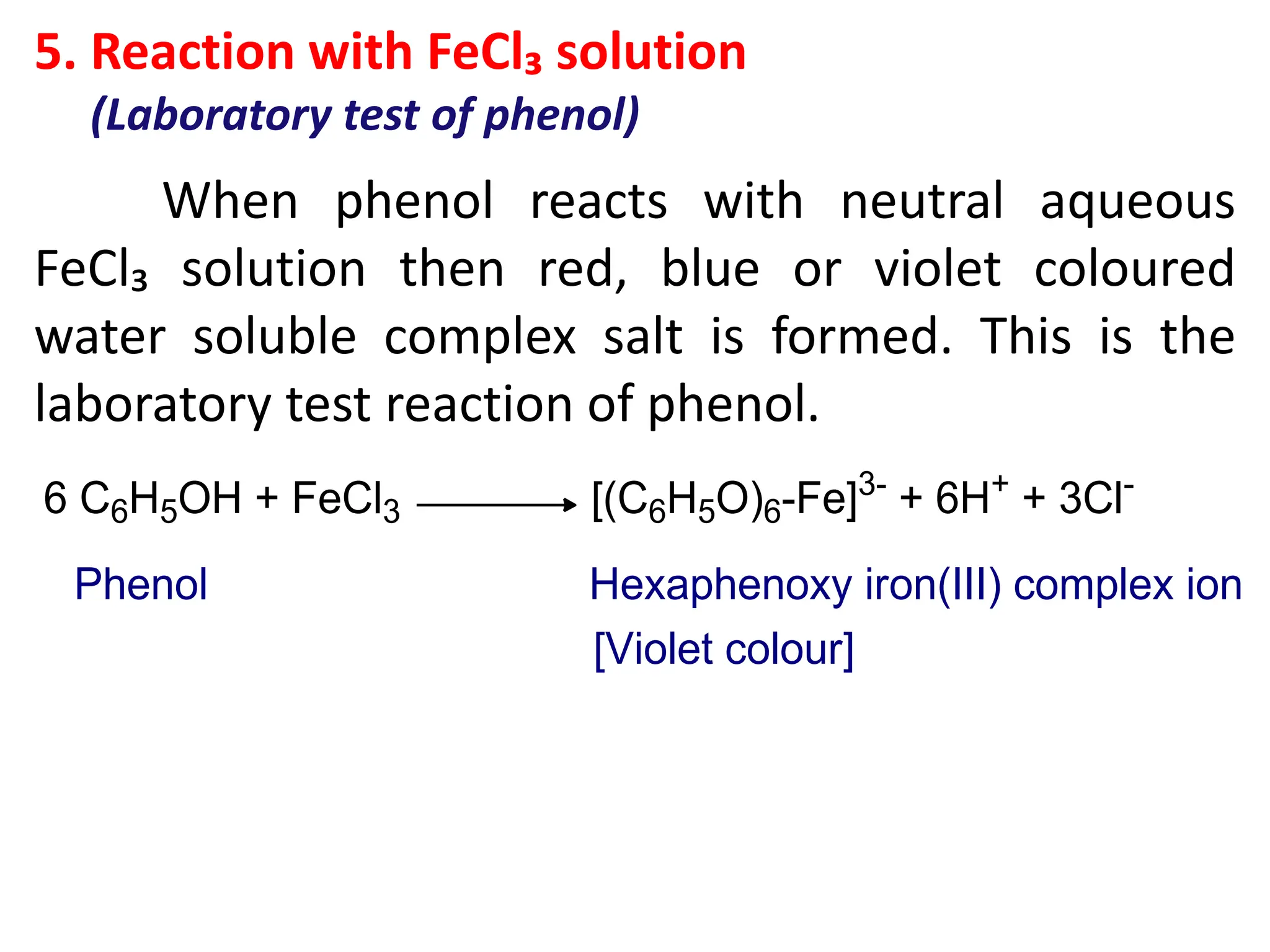
![Chemical properties of phenol
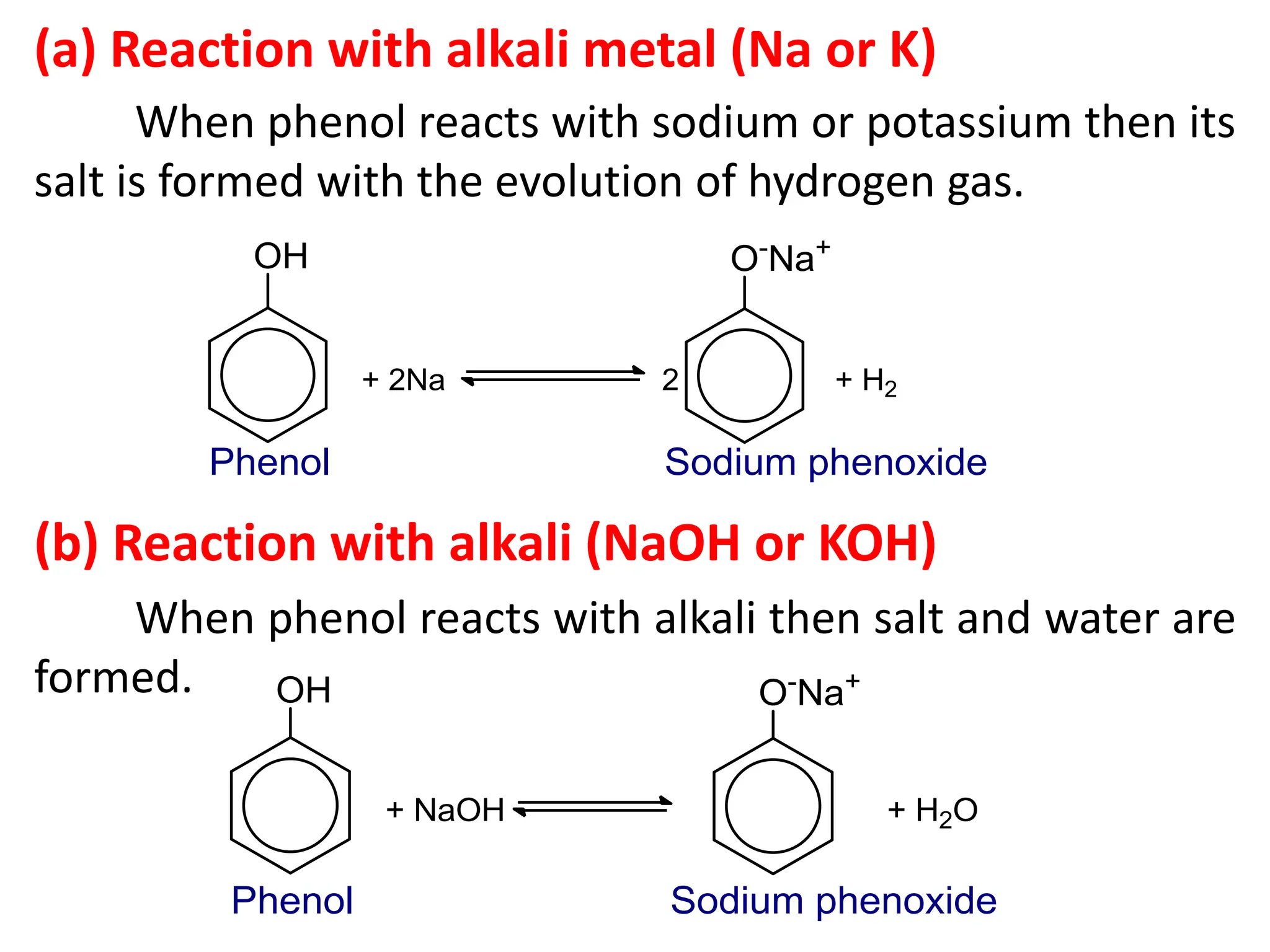
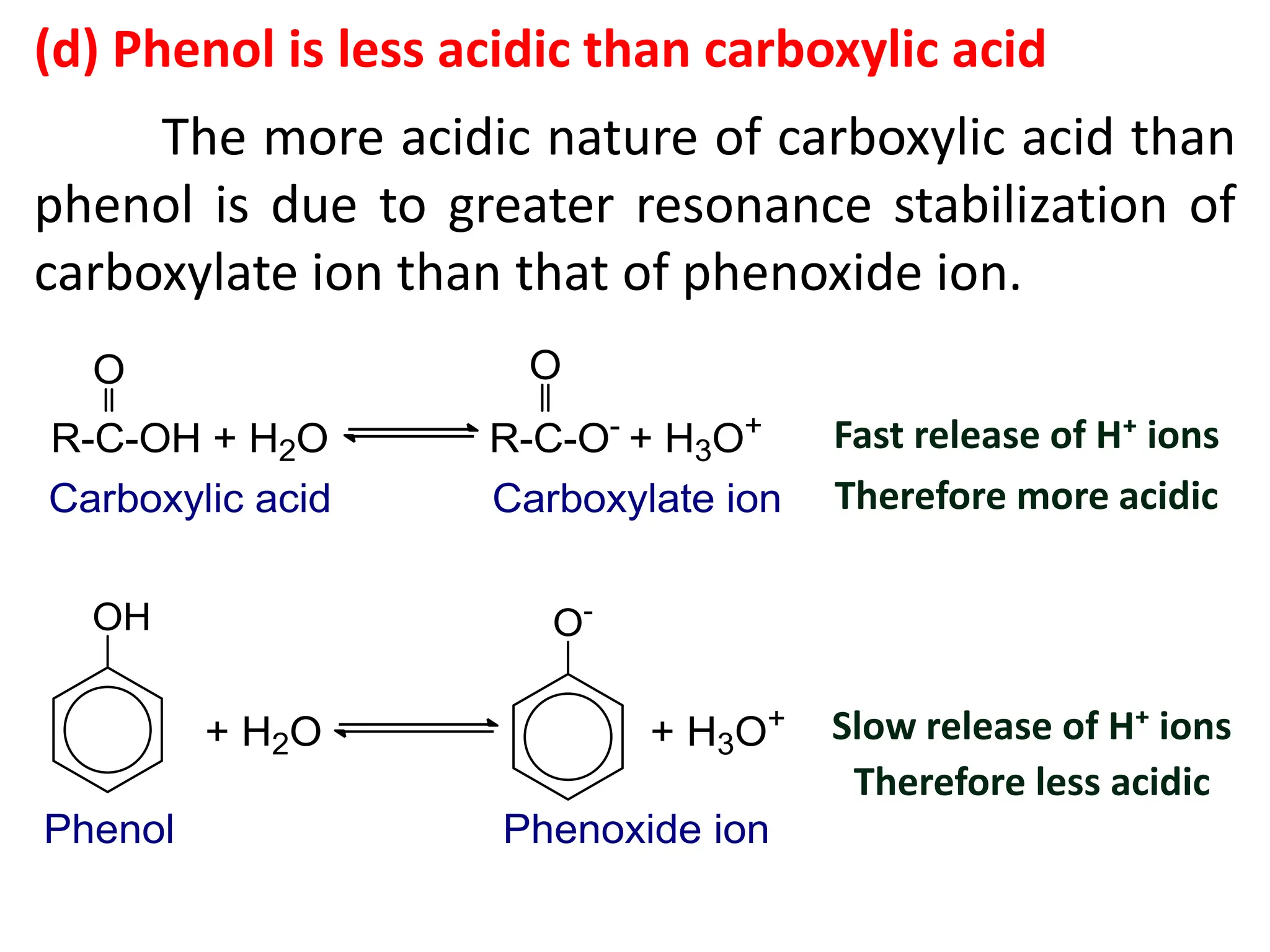
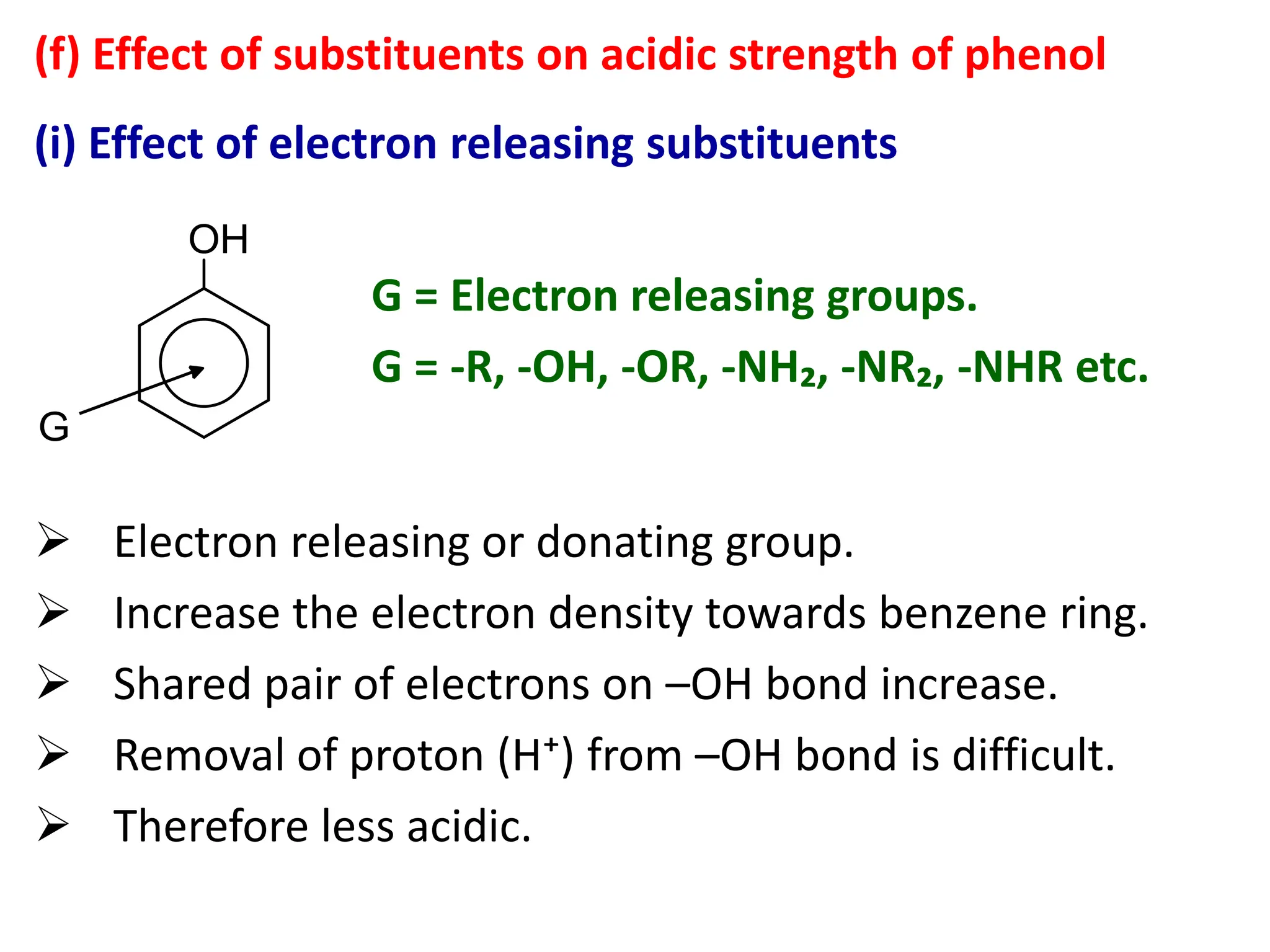
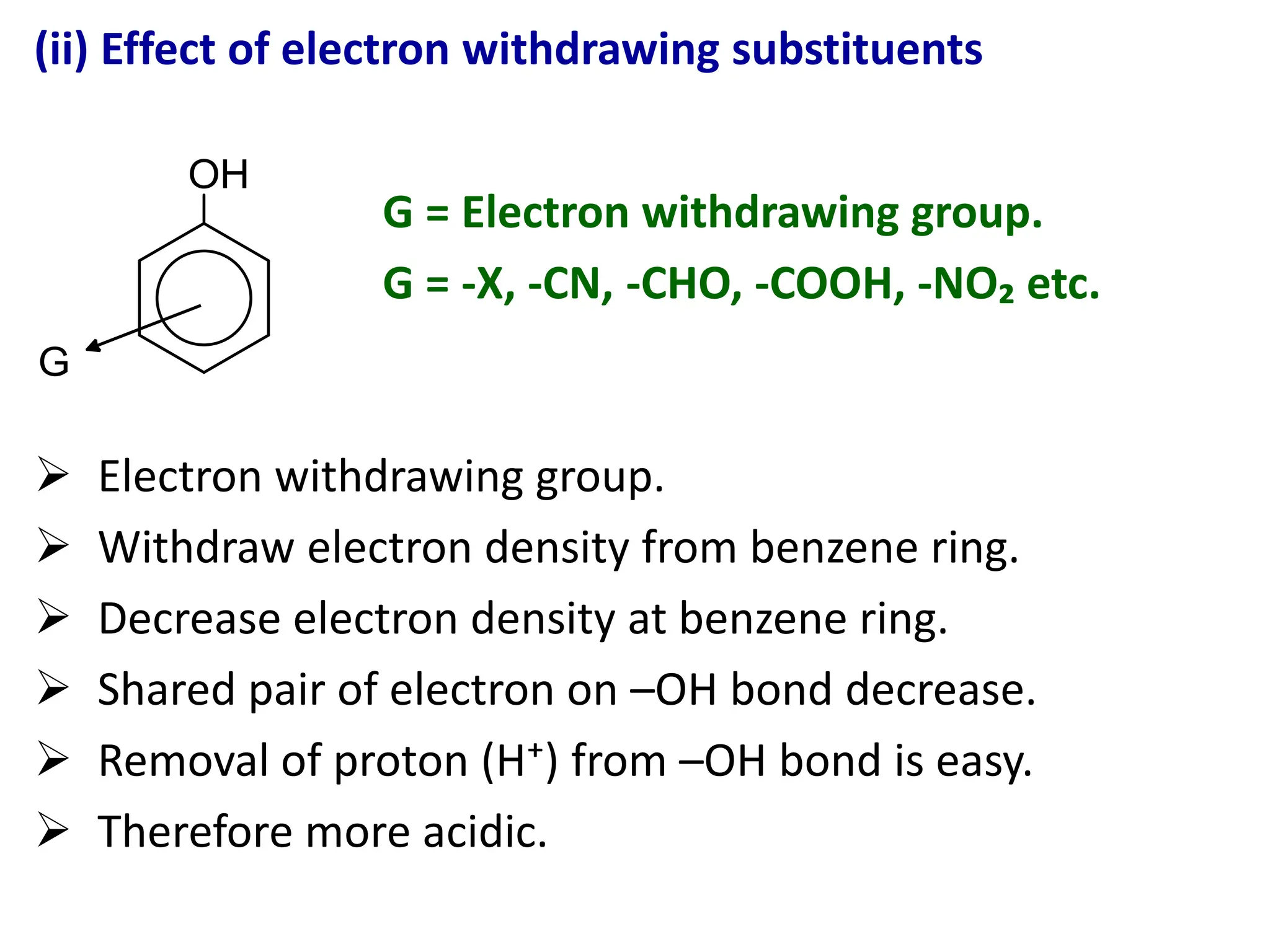
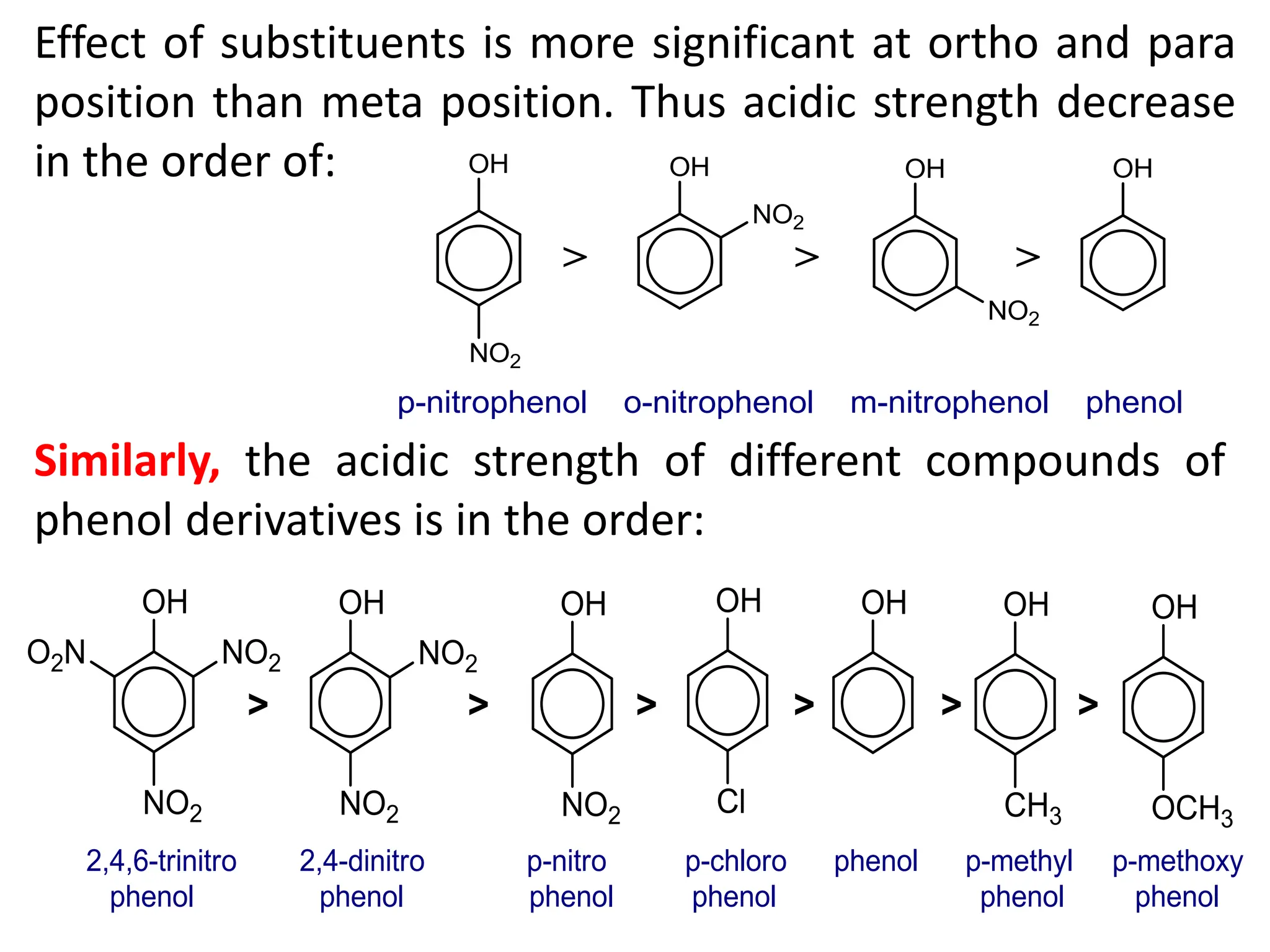
1. Acidic nature of phenol
Phenol is weakly acidic in nature because it
slightly ionize in aqueous solution to give phenoxide
ion and H⁺ or H₃O⁺ ions.
Phenol turns blue litmus paper to red which indicates
acidic nature.
[A] Reaction due to –OH group of phenol](https://image.slidesharecdn.com/4-241130161016-baa03818/75/4-Phenols-pdf-which-explain-about-the-general-methods-of-preparation-of-phenols-physical-and-chemical-properties-and-it-s-uses-10-2048.jpg)
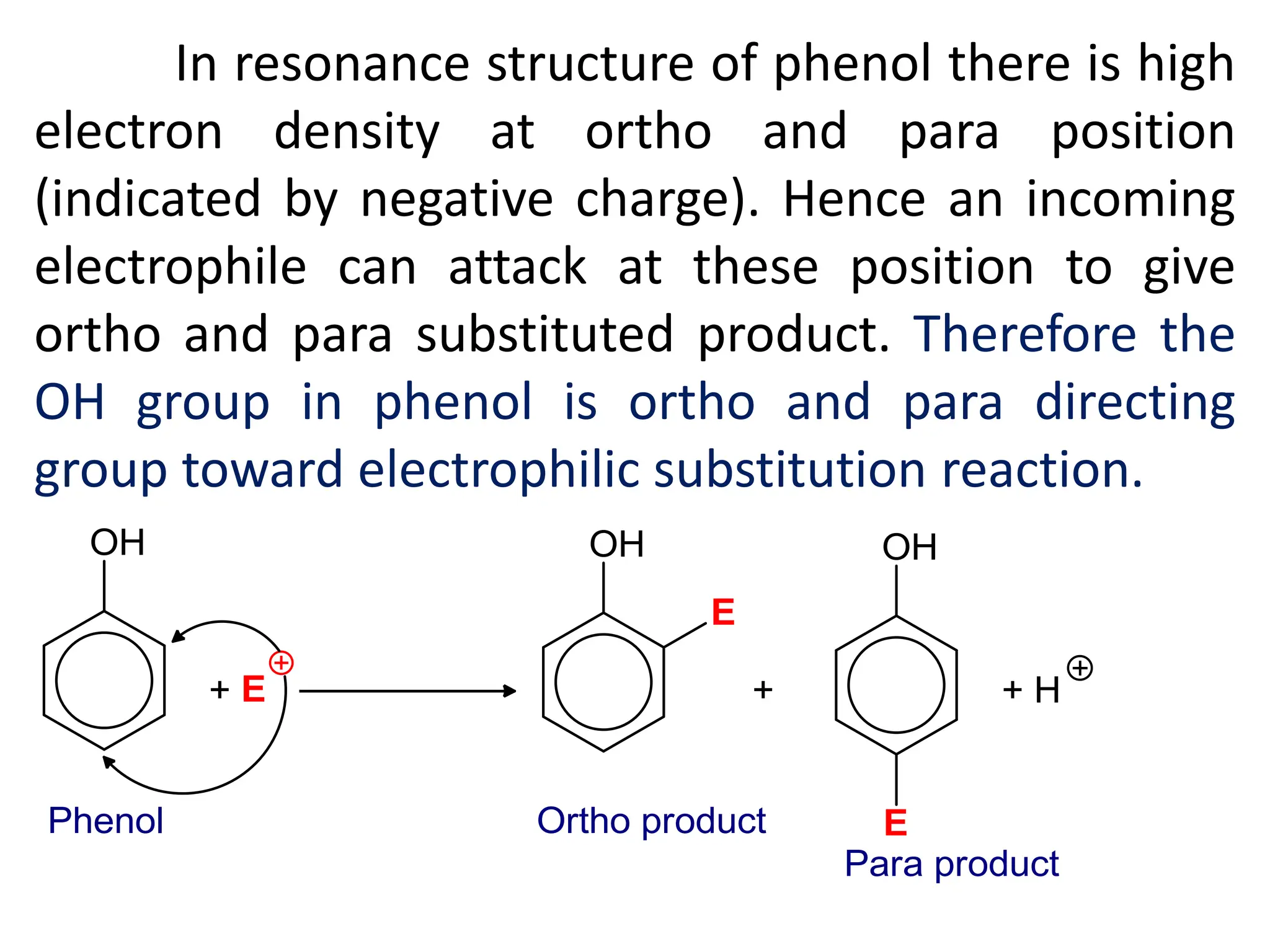
![[B] Reaction due to aromatic ring
(Electrophilic substitution reaction)
The –OH group in phenol is electron releasing
group that increase electron density at benzene ring
and benzene ring becomes more reactive. Therefore
the –OH group in phenol is ring activator group due
to positive resonance effect (+R effect).](https://image.slidesharecdn.com/4-241130161016-baa03818/75/4-Phenols-pdf-which-explain-about-the-general-methods-of-preparation-of-phenols-physical-and-chemical-properties-and-it-s-uses-22-2048.jpg)
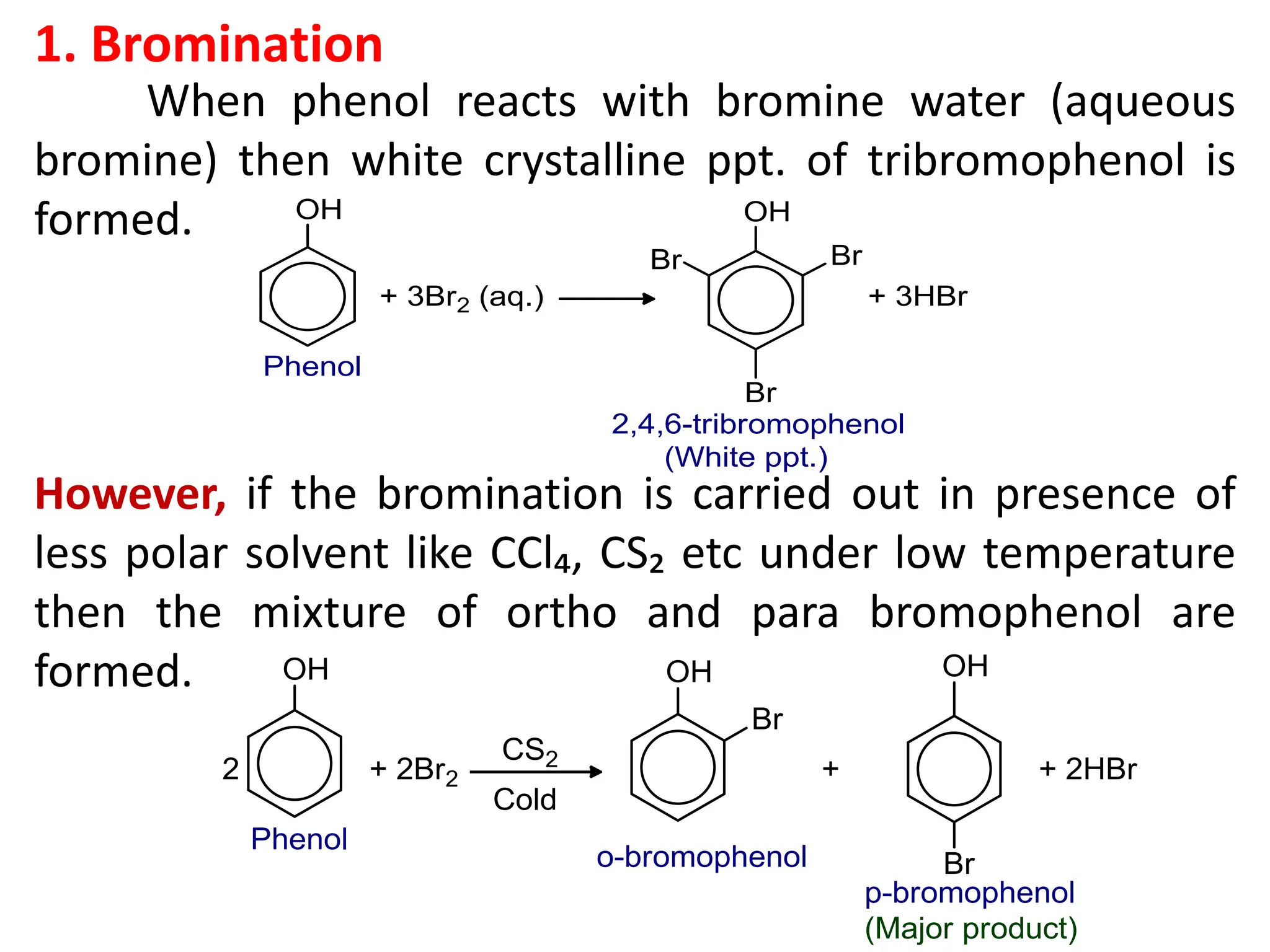
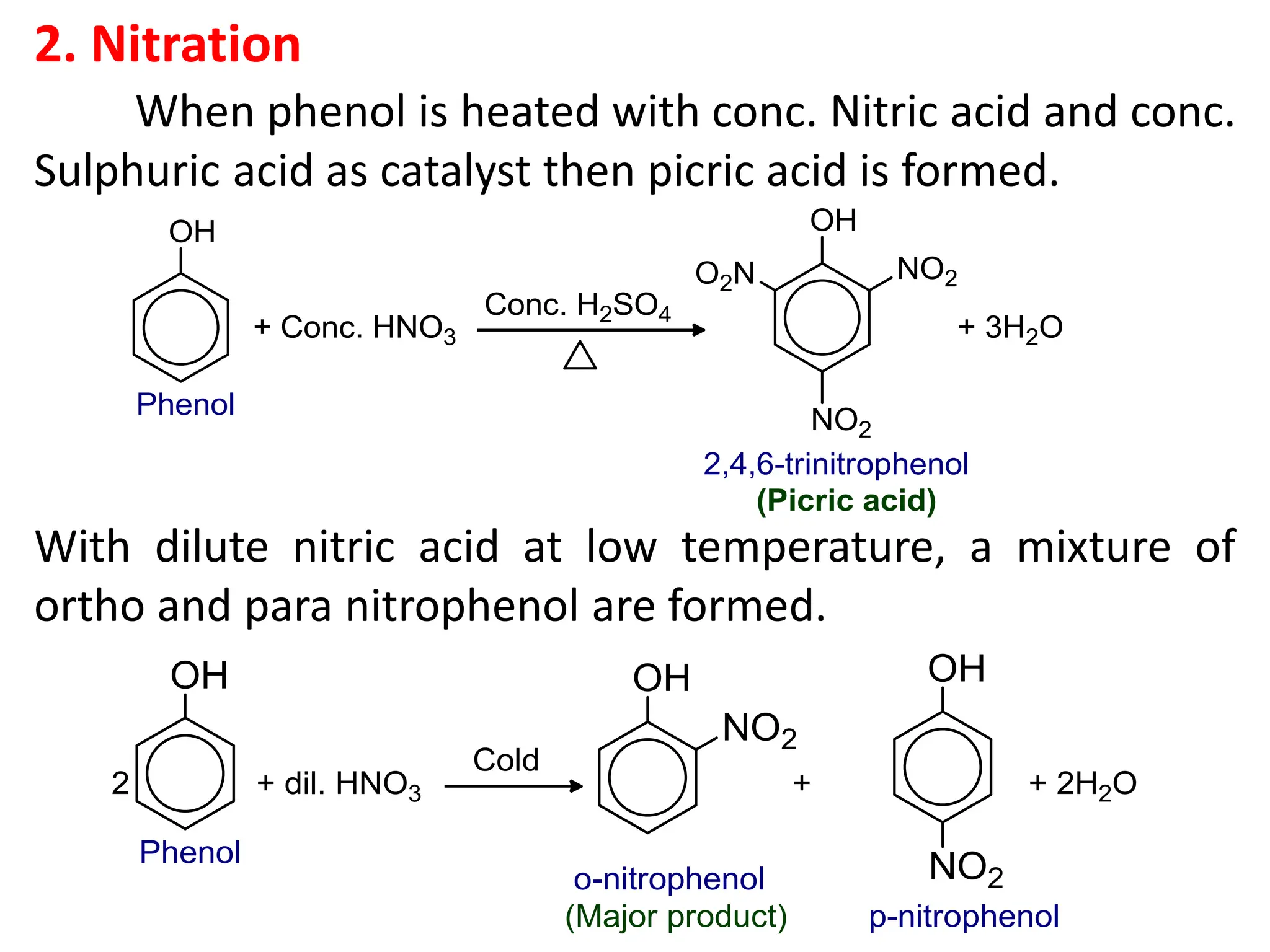
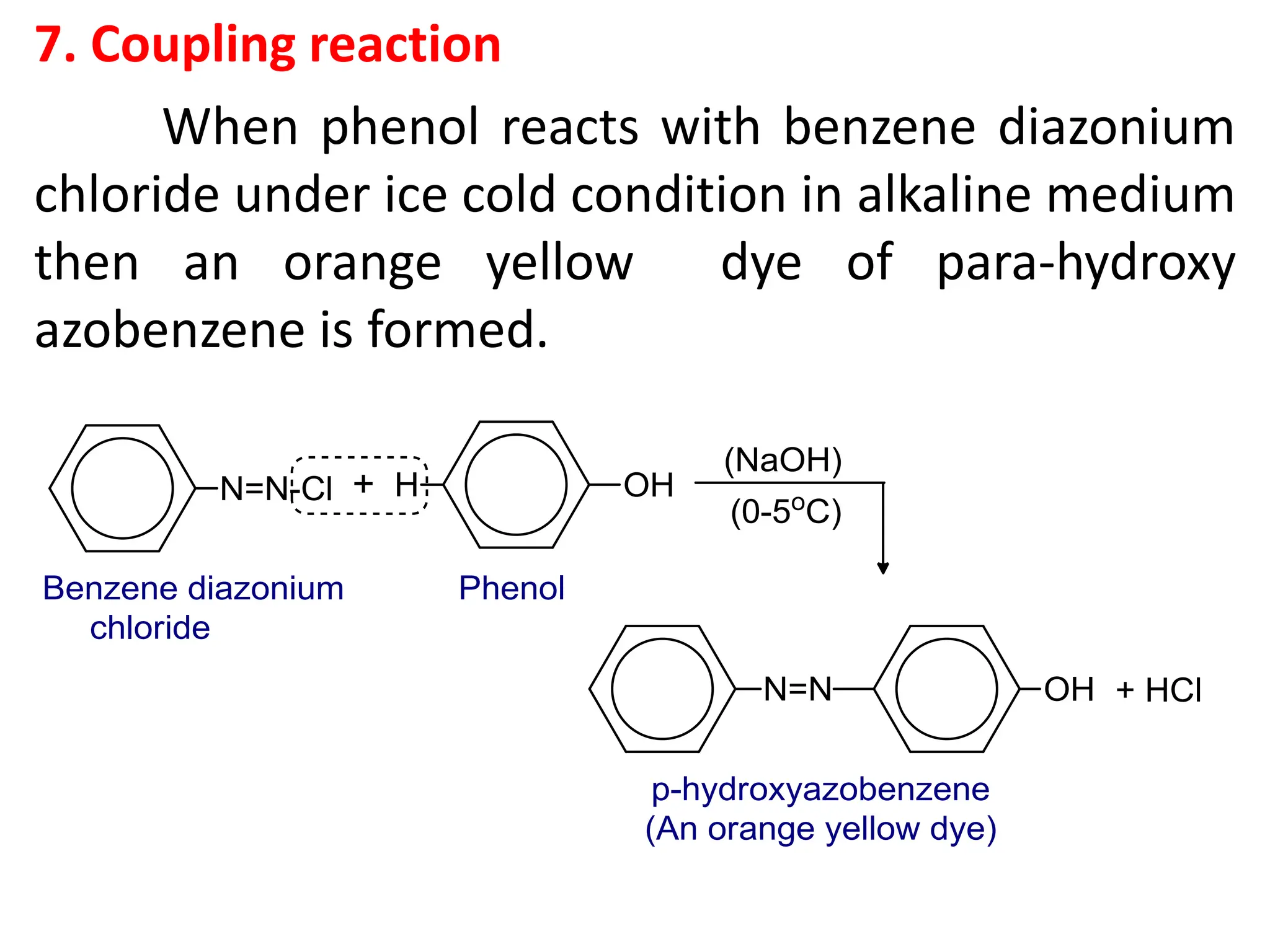
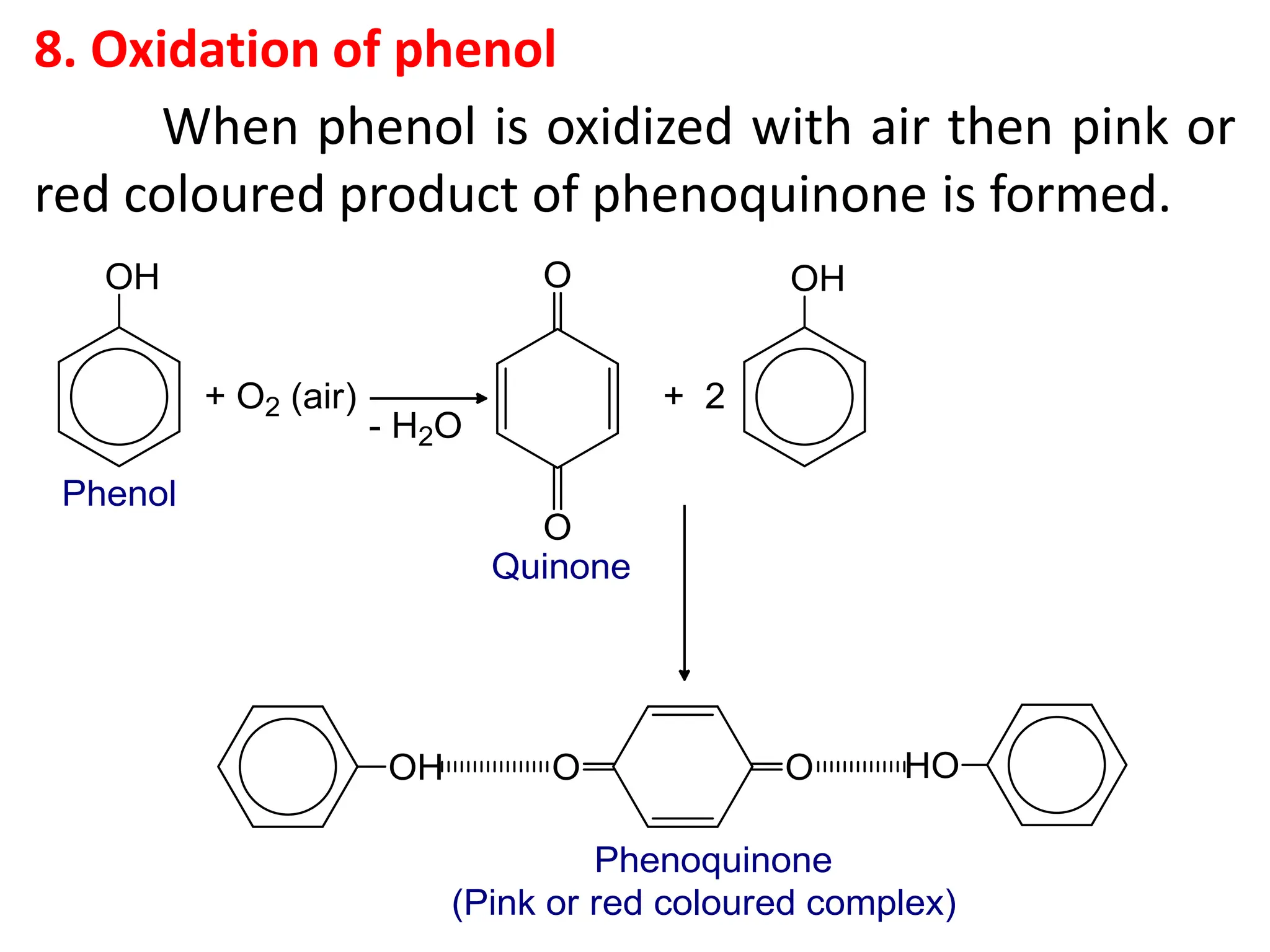
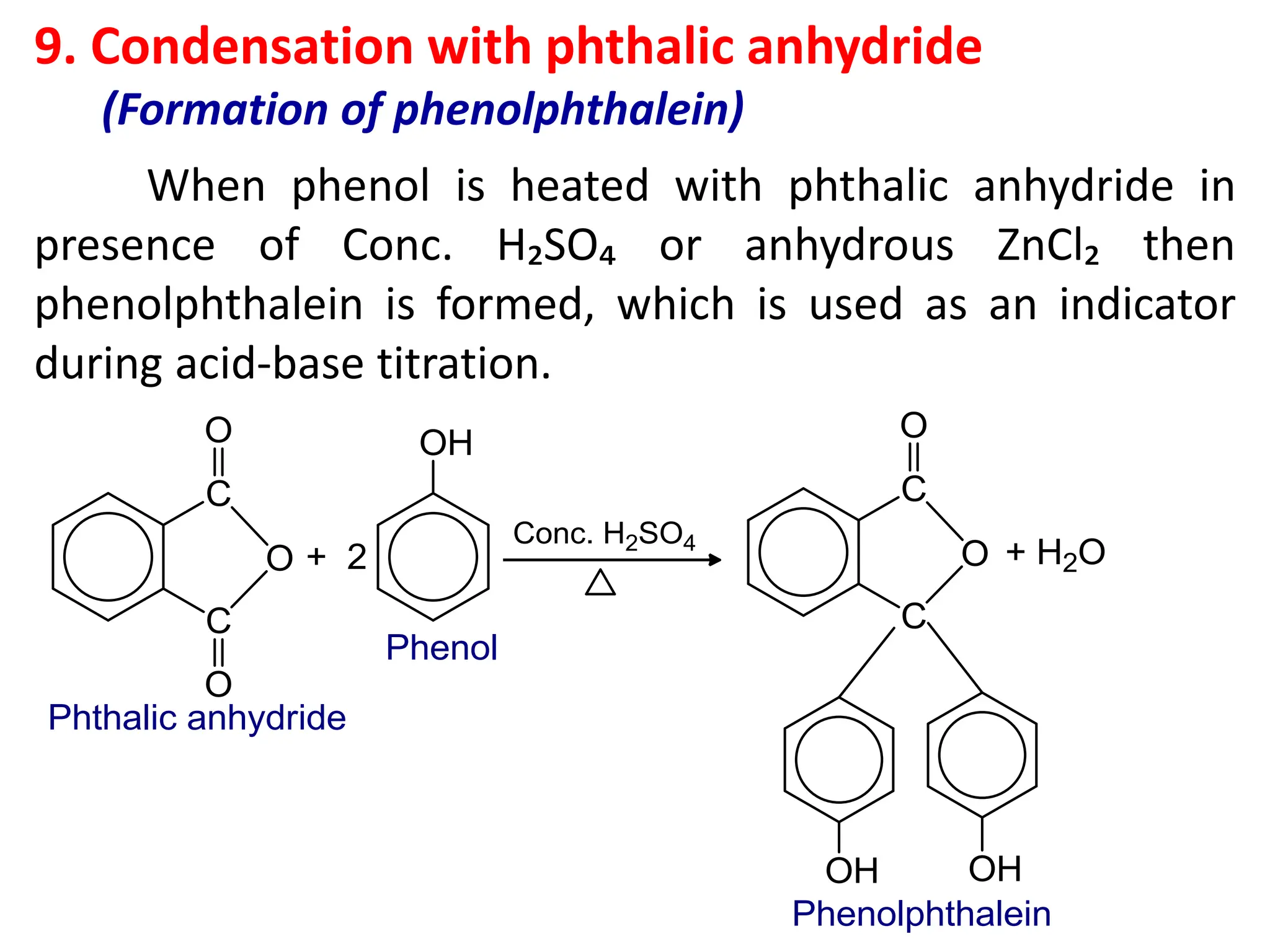
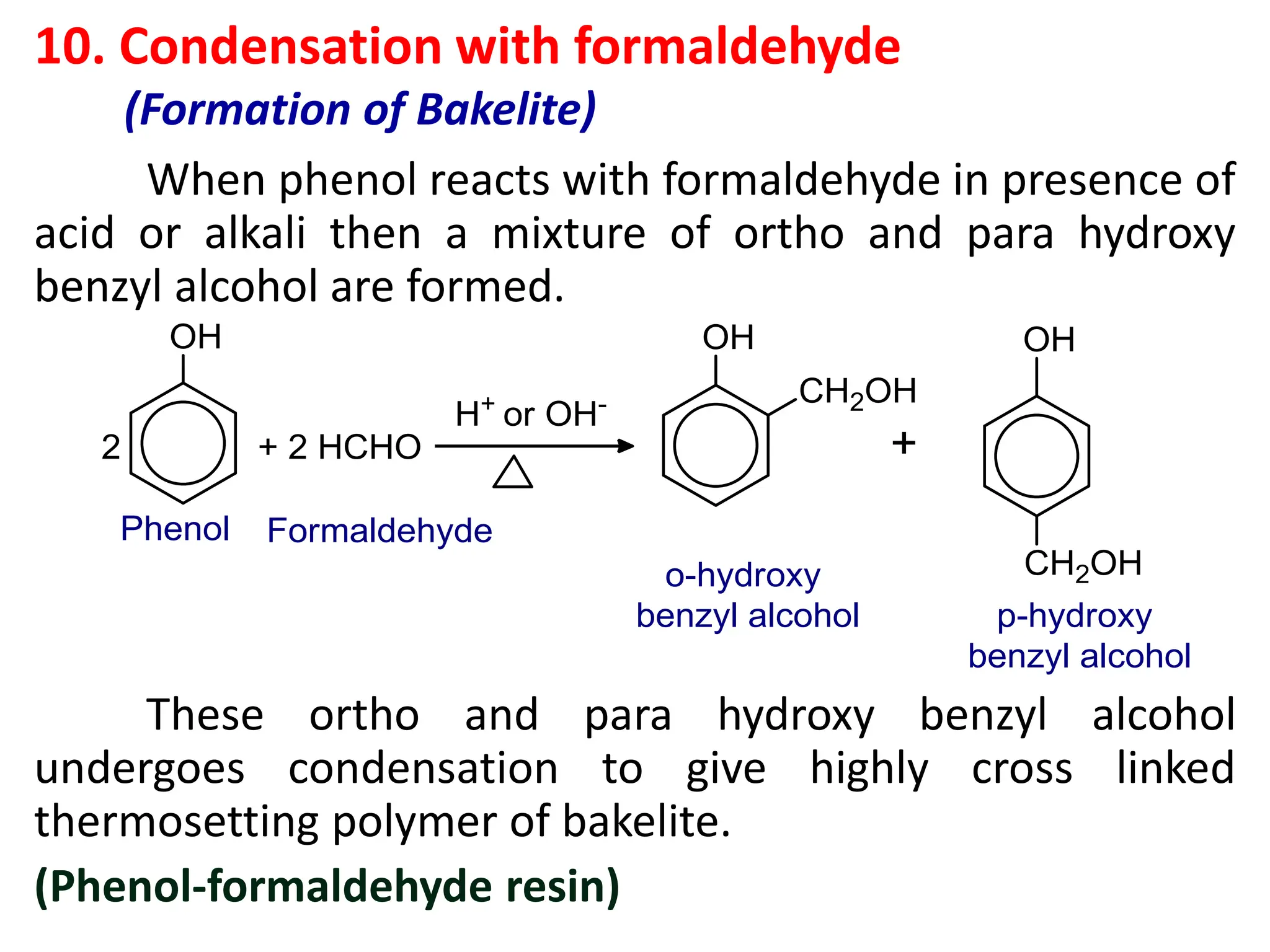
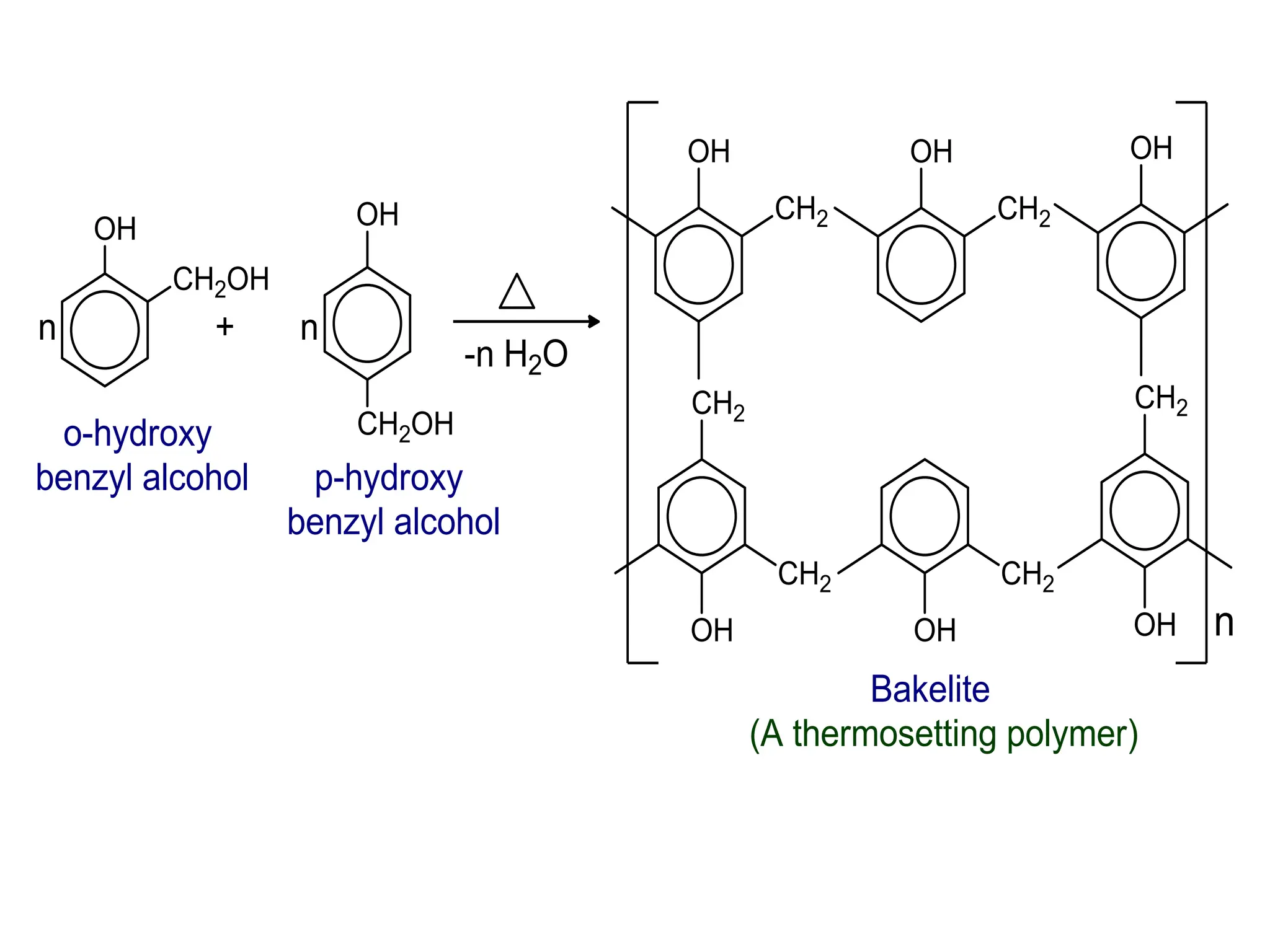
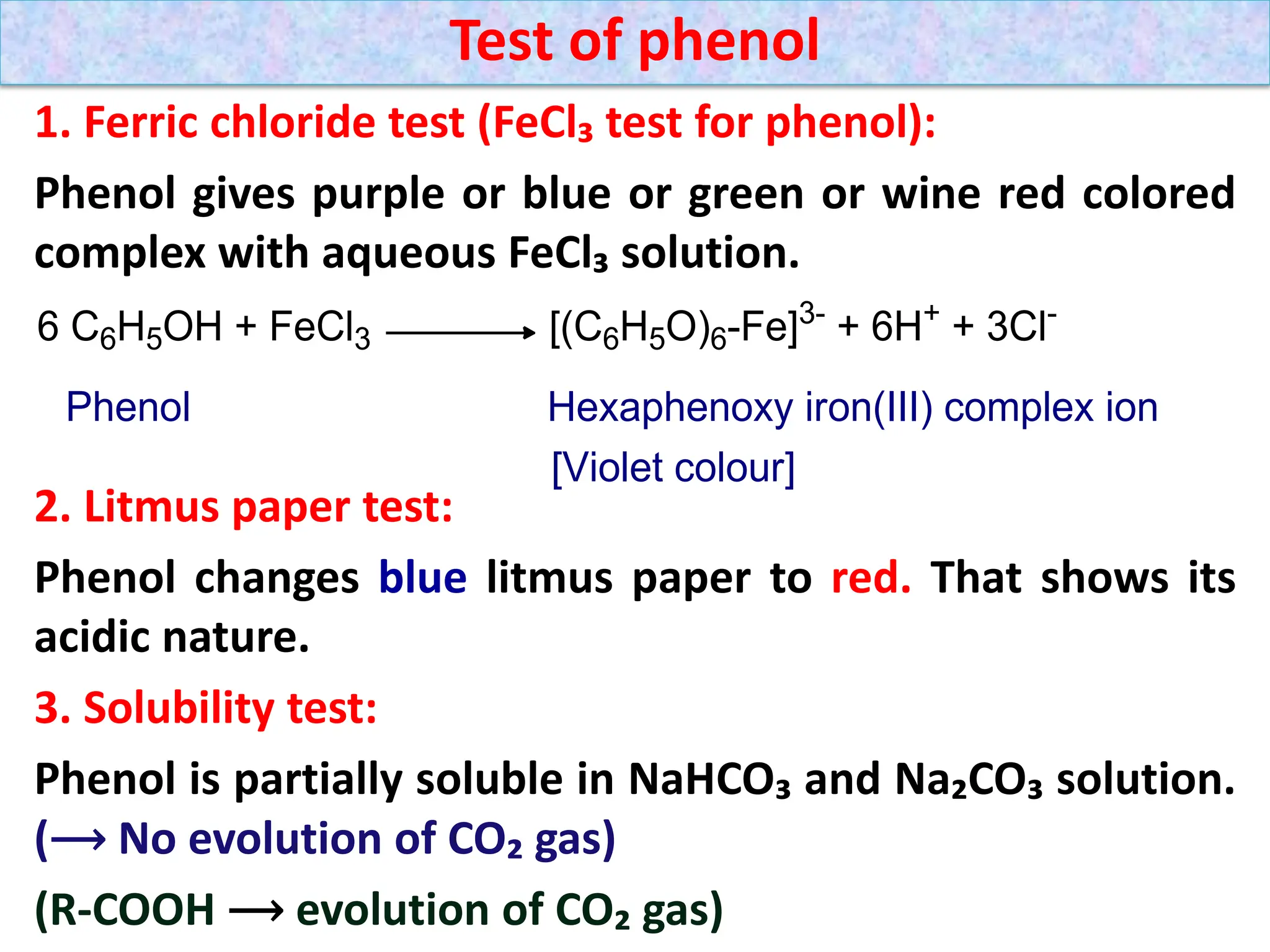
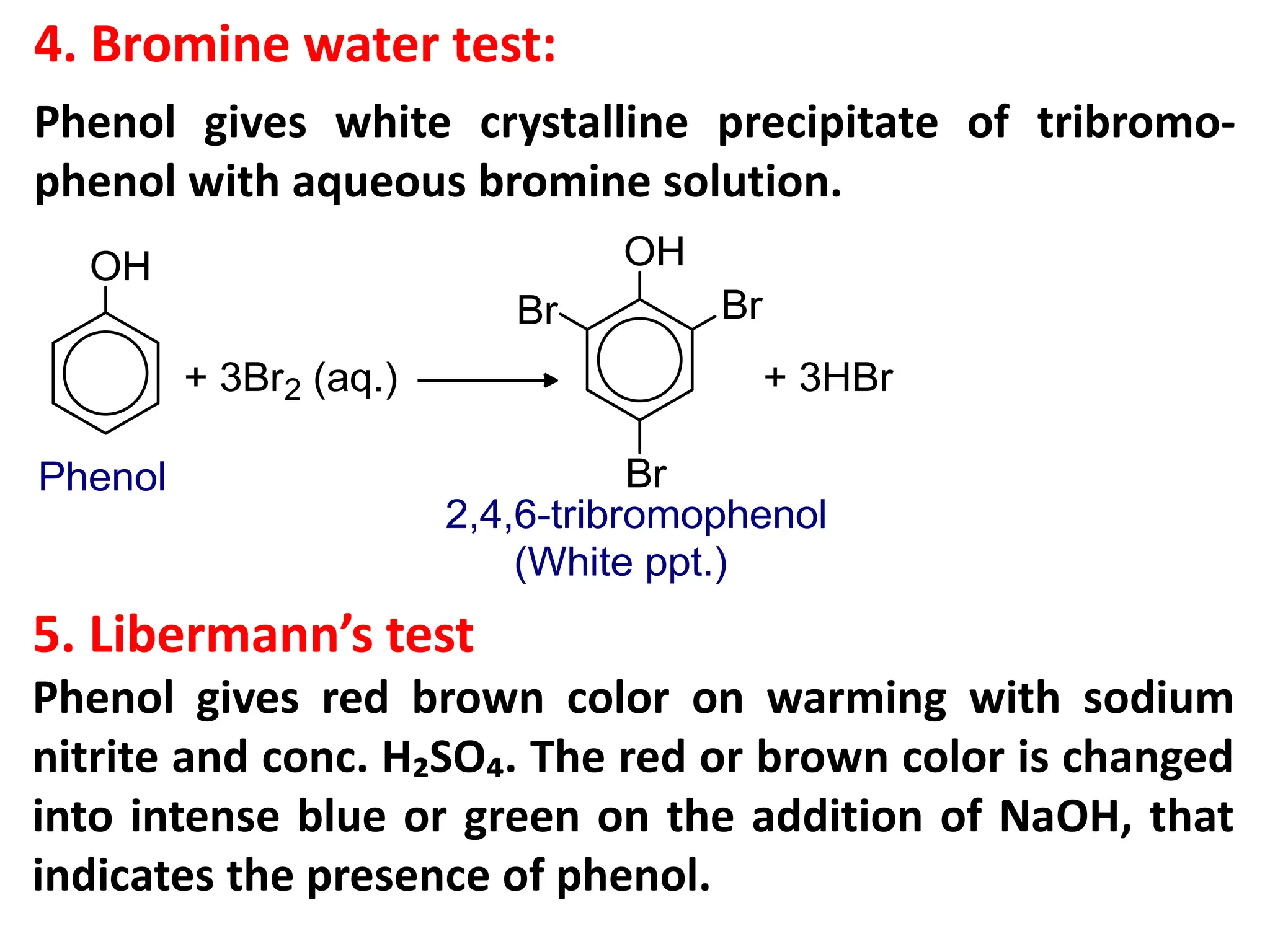
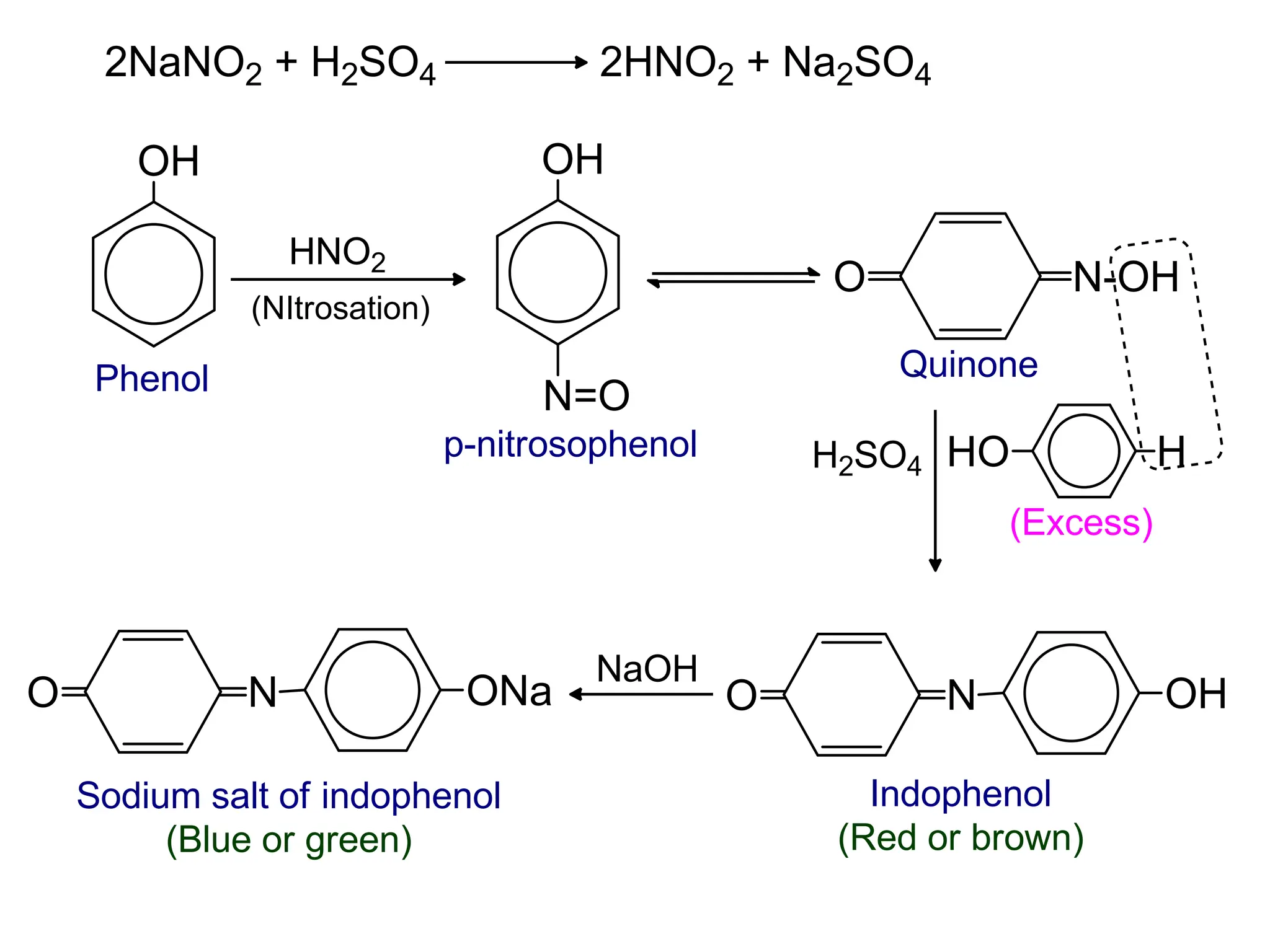



![1. Write any three general methods of preparation of
phenol. What is the test reaction of phenol? [3+2]
2. How is phenol prepared from (a) benzene sulphonic
acid (b) aniline (c) chlorobenzene. How would you
explain the –OH group in phenol is ortho para directing
group toward electrophilic substitution reaction? [3+2]
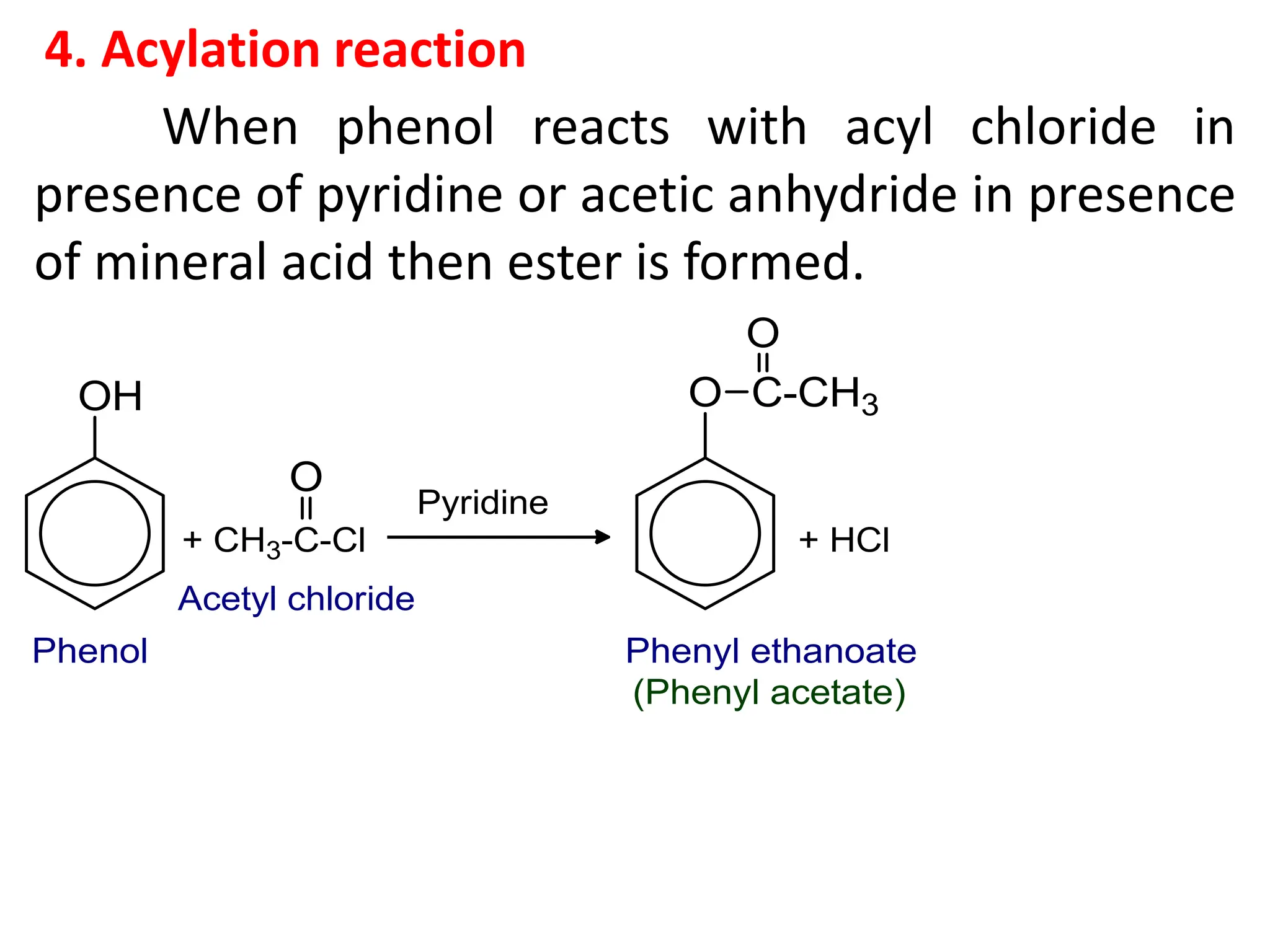
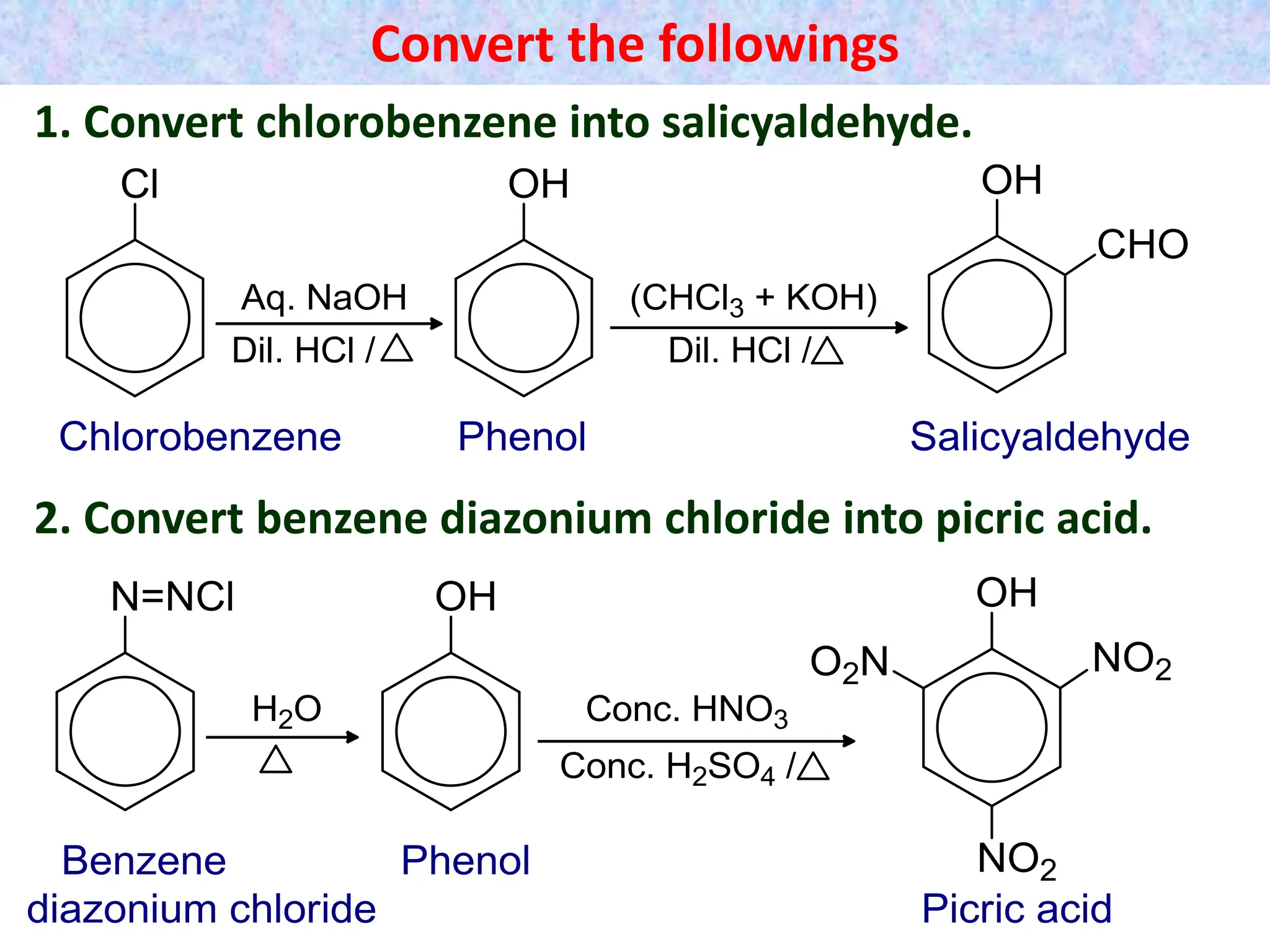
3. Starting from phenol, how would you prepare (a) p-
hydroxyazobenzene (b) salicyaldehyde (c) phenyl
acetate (d) salicylic acid (e) p-nitrophenol. [1x5=5]
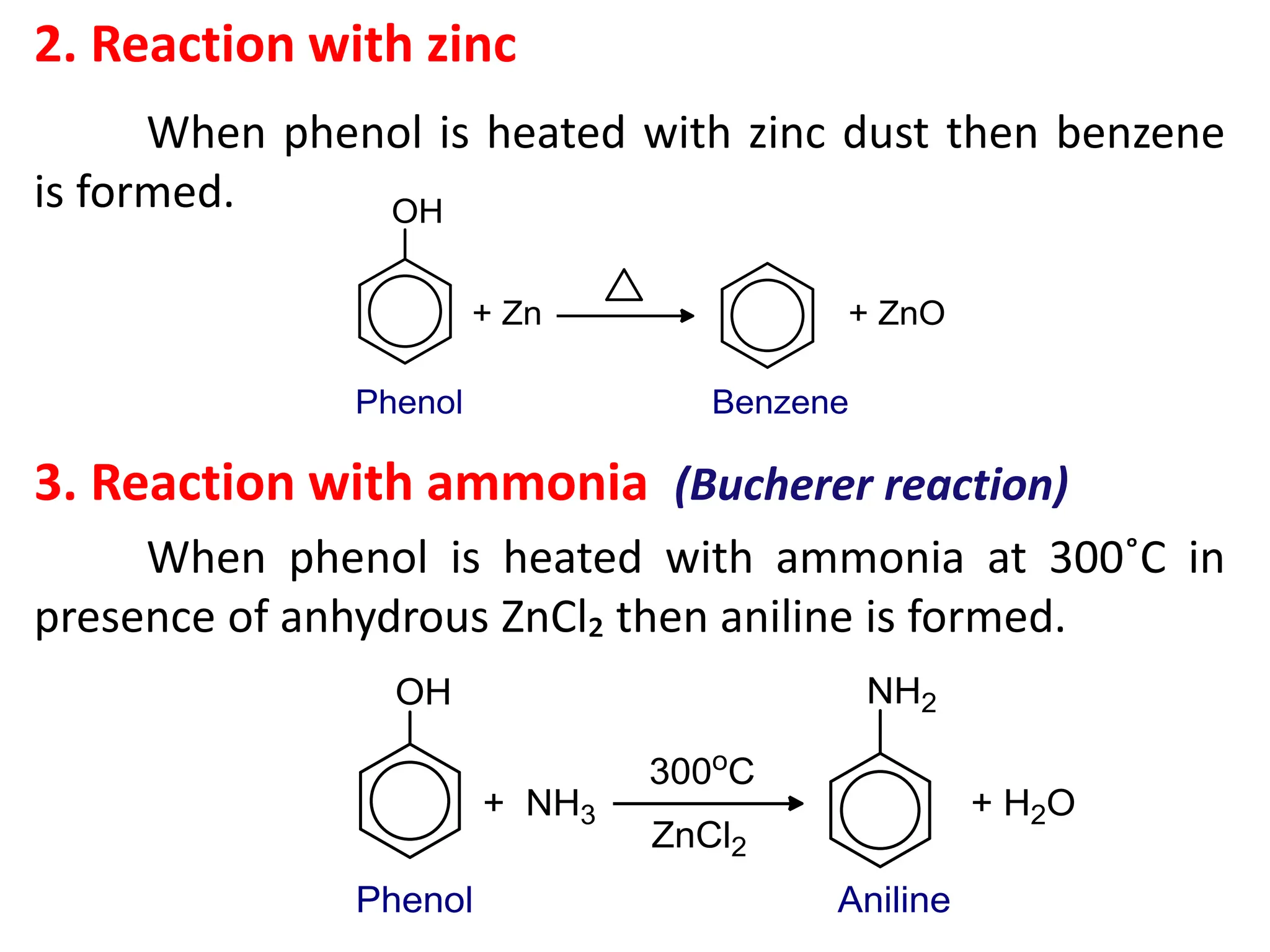
4. What happens when phenol is treated with (a) Aqueous
bromine (b) FeCl₃ solution (c) dilute HNO₃ (d) heated
zinc dust (e) Conc. HNO₃ and H₂SO₄? [1x5=5]
Some Important questions for exam (Short Questions)](https://image.slidesharecdn.com/4-241130161016-baa03818/75/4-Phenols-pdf-which-explain-about-the-general-methods-of-preparation-of-phenols-physical-and-chemical-properties-and-it-s-uses-45-2048.jpg)
![1. Identify major product A, B, C and D in the following
reaction sequence.
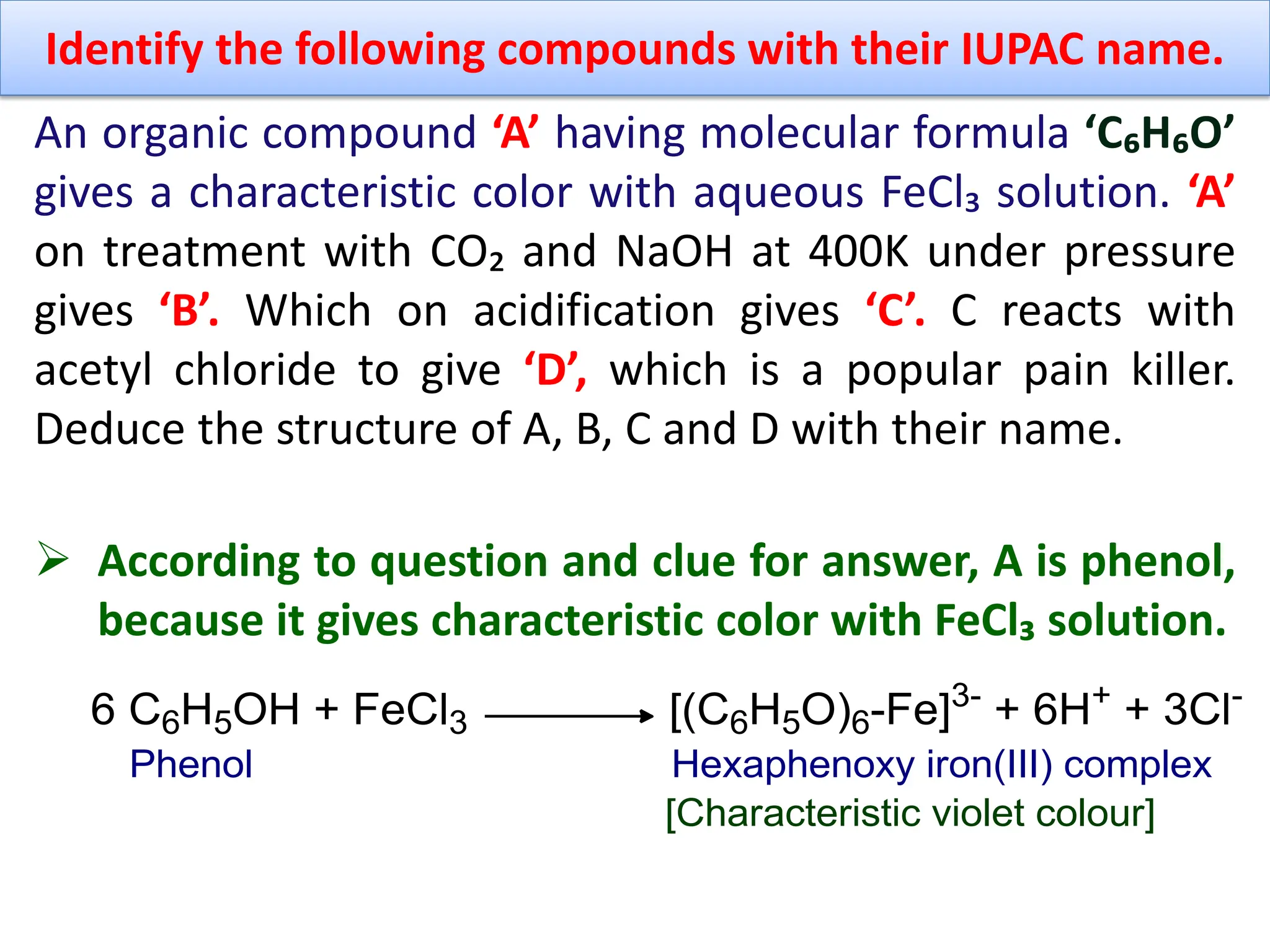
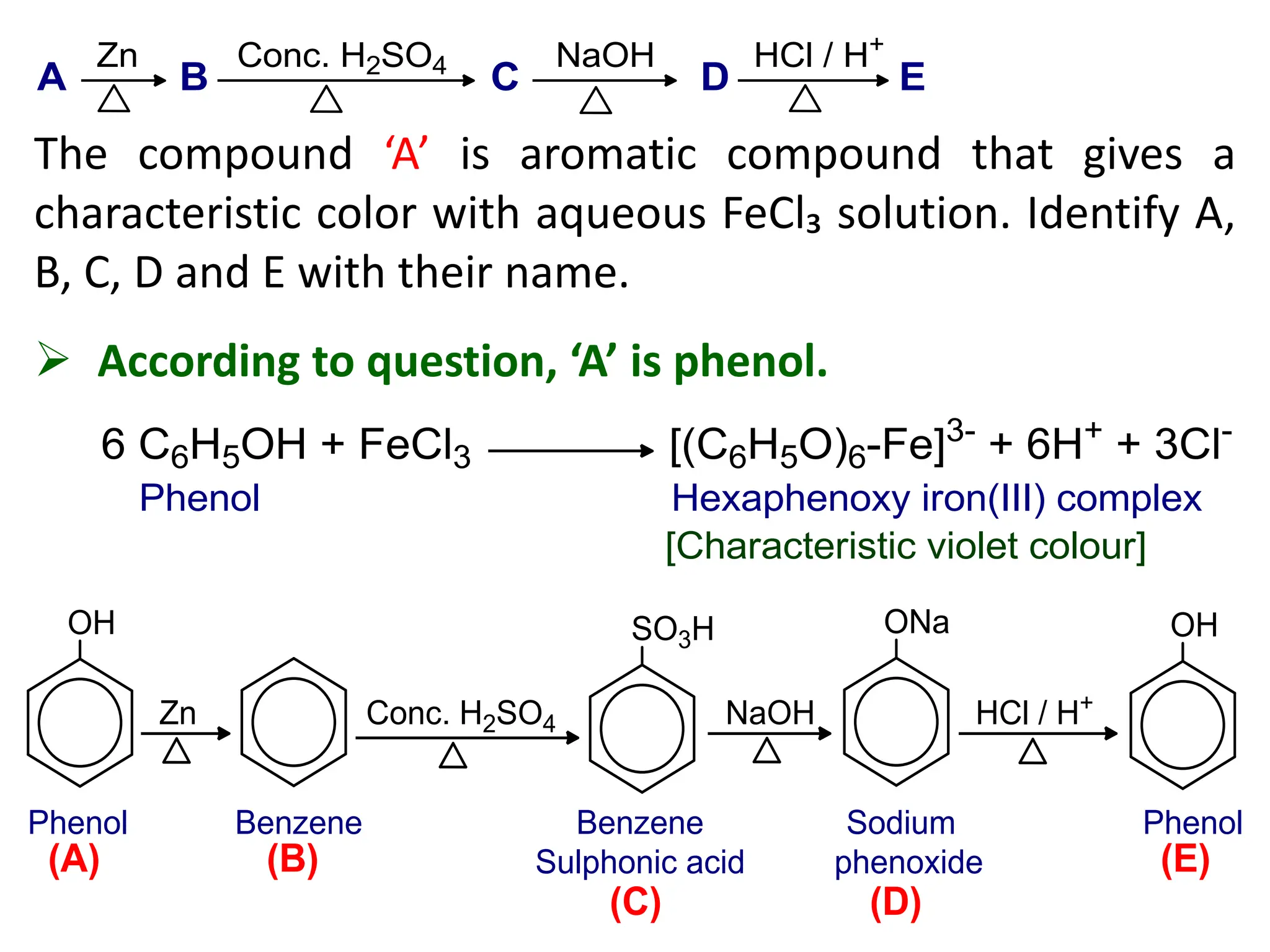
2. An aromatic compound ‘A’ gives parent hydrocarbon ‘B’
when heated with Zn dust. The compound B can also be
obtained by polymerization of acetylene. The
compound A gives characteristic violet color with FeCl₃
solution. Identify A and B with their name and suitable
chemical reaction. Why the compound A is slightly
acidic in nature? [4+1]
The compound D gives toluene with zinc amalgam in
presence of HCl. [4+1]](https://image.slidesharecdn.com/4-241130161016-baa03818/75/4-Phenols-pdf-which-explain-about-the-general-methods-of-preparation-of-phenols-physical-and-chemical-properties-and-it-s-uses-46-2048.jpg)
![Some Important questions for exam (Long Questions)
Phenol is an important starting material for the
preparation of large number of industrial materials, out
of them picric acid is important explosive substance.
(a) Write the principle reaction for the preparation of
phenol from chlorobenzene.
(b) How phenol can also be obtained from benzene
diazonium chloride?
(c) How picric acid is obtained from phenol? Write any use
of picric acid.
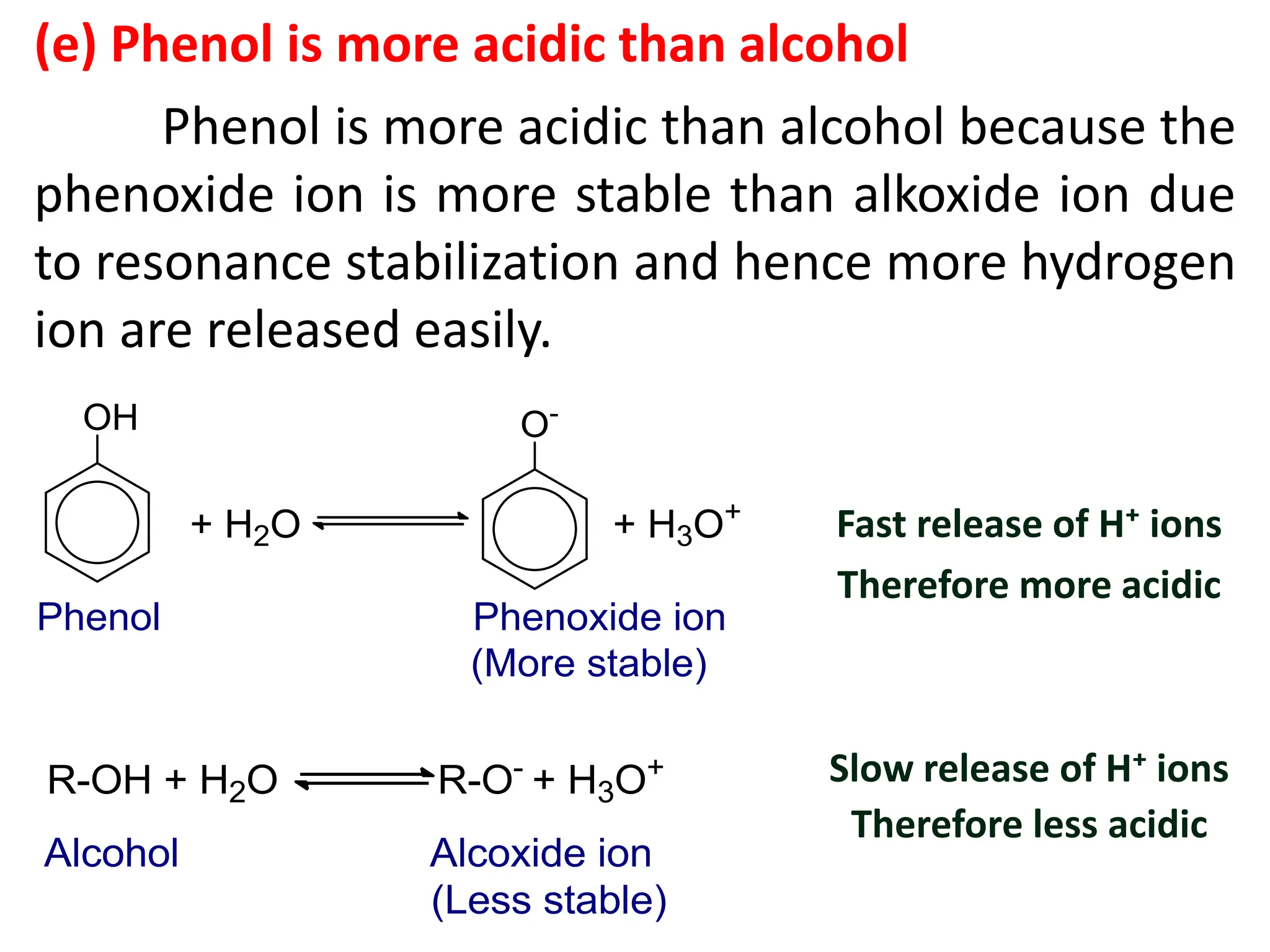
(d) How phenol is slightly acidic in nature? Compare the
acidic strength of phenol with alcohol.
(e) Write any two important uses of phenol.
[2+1+2+2+1]](https://image.slidesharecdn.com/4-241130161016-baa03818/75/4-Phenols-pdf-which-explain-about-the-general-methods-of-preparation-of-phenols-physical-and-chemical-properties-and-it-s-uses-47-2048.jpg)
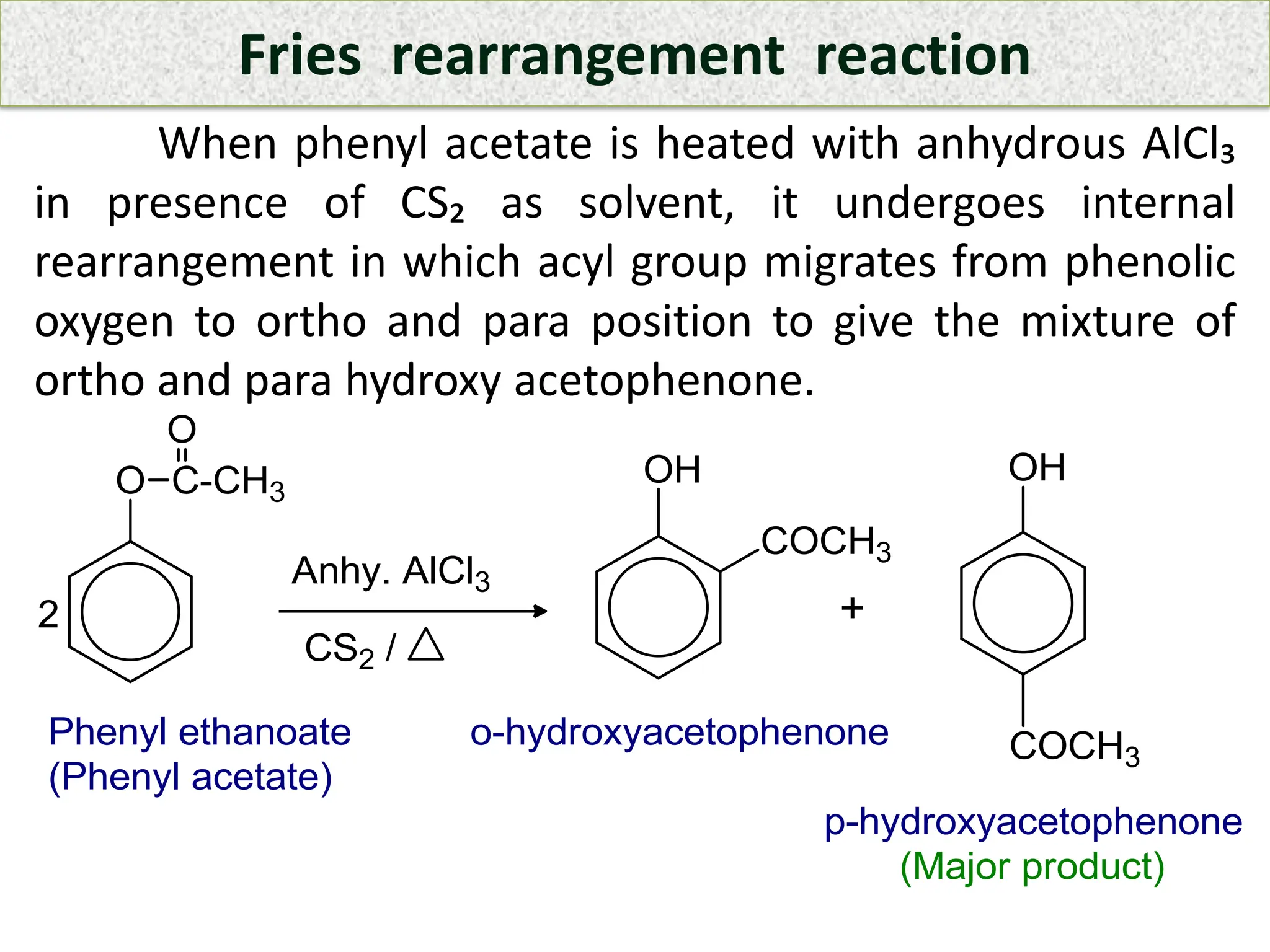
![ An aromatic compound ‘A’ on diazotization gives
compound ‘B’ which is warmed with water to give
compound ‘C’. The compound C is heated with acetyl
chloride in presence of pyridine gives ‘D’ which
undergoes internal rearrangement in presence of
anhydrous AlCl₃ and CS₂ as solvent gives a mixture of ‘E’
and ‘F’. The compound C is heated with zinc dust to give
parent hydrocarbon benzene. Identify A, B, C, D, E and F
with their name and suitable chemical reaction involved
for it.
(a) What happens when compound C is heated with conc.
HNO₃ and conc. H₂SO₄?
(b) Write any two uses of Compound C.
[6+1+1]](https://image.slidesharecdn.com/4-241130161016-baa03818/75/4-Phenols-pdf-which-explain-about-the-general-methods-of-preparation-of-phenols-physical-and-chemical-properties-and-it-s-uses-48-2048.jpg)
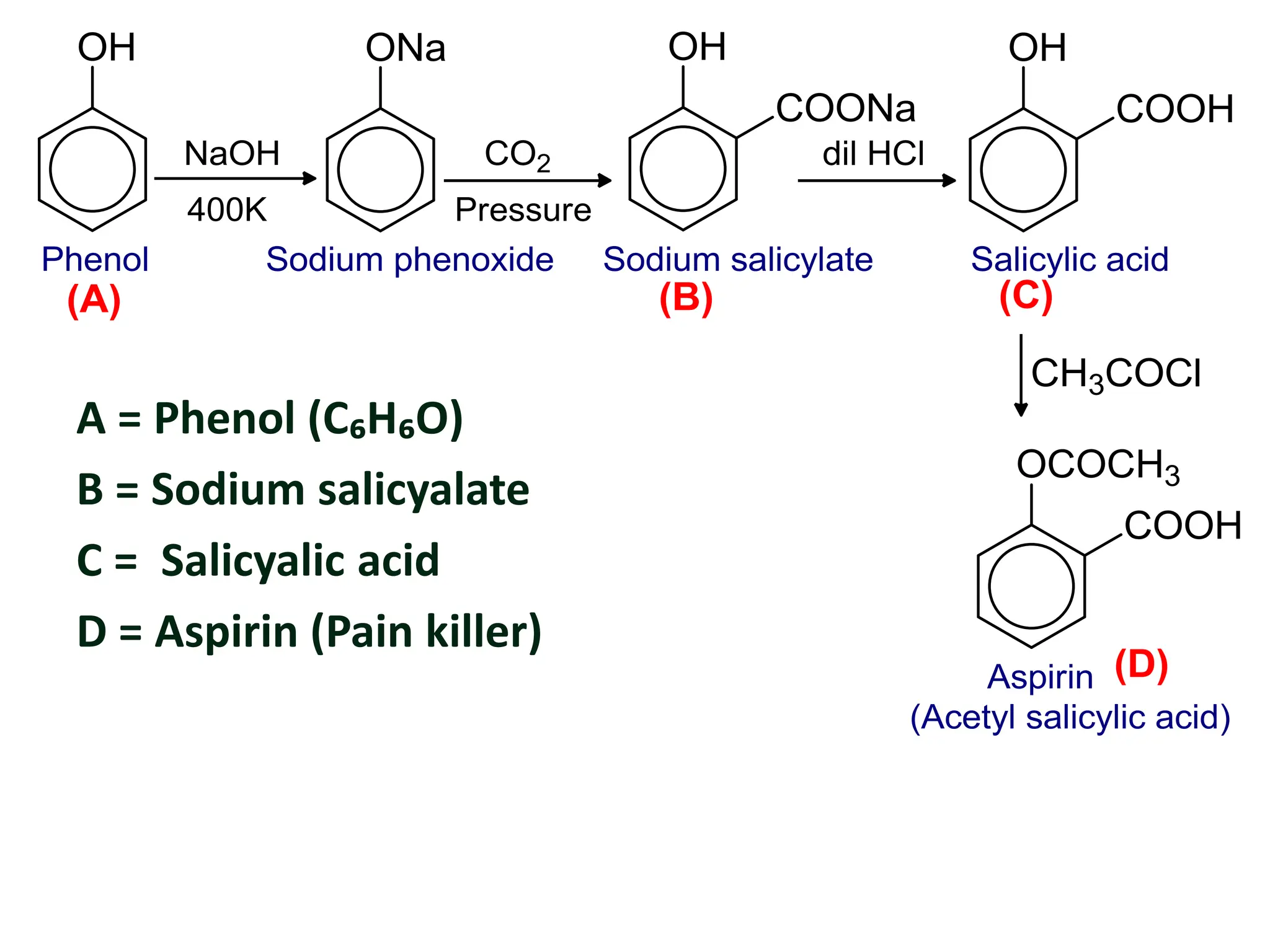
![ An aromatic hydrocarbon ‘A’ reacts with sodium
carbonate to give ‘B’ which is heated with solid NaOH at
about 300⁰C gives ‘C’. On acidification of C it gives ‘D’.
The compound D reacts with benzene diazonium
chloride to give ‘E’ which is an important dye. The
compound D is an aromatic compound having
molecular formula ‘C₆H₆O’ which give characteristic
violet color with FeCl₃ solution and is also obtained by
warming benzene diazonium chloride with water.
Identify A, B, C, D and E with their name and suitable
chemical reaction.
(a) What happens when compound D reacts with aq. Br₂?
(b) What is the action of compound D with blue litmus
paper? [6+1+1]](https://image.slidesharecdn.com/4-241130161016-baa03818/75/4-Phenols-pdf-which-explain-about-the-general-methods-of-preparation-of-phenols-physical-and-chemical-properties-and-it-s-uses-49-2048.jpg)
