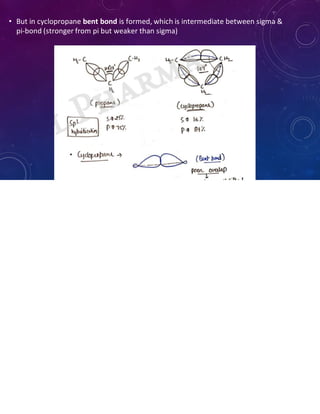
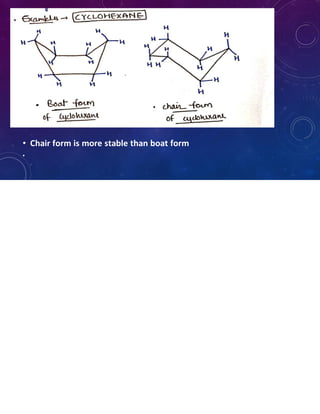
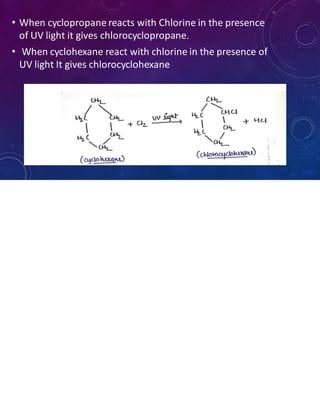
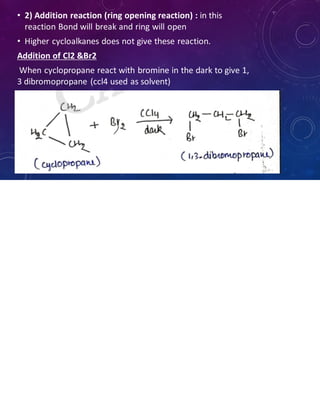
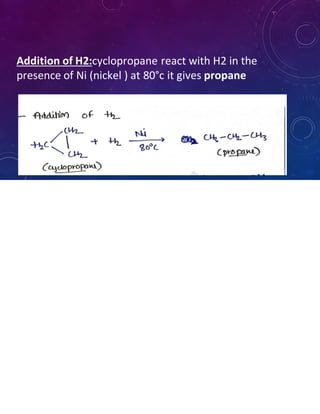
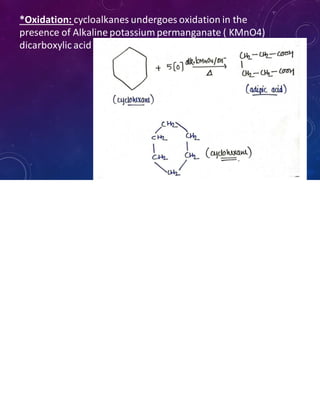
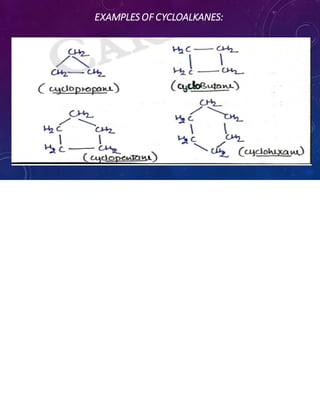
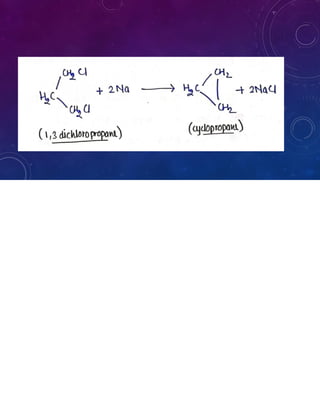
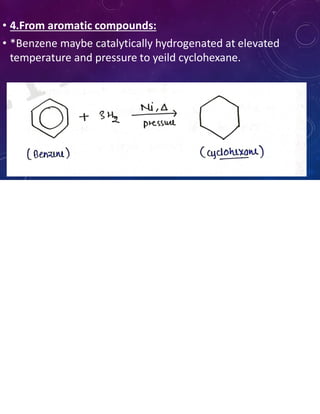
This document provides an overview of cycloalkanes. It begins with definitions and examples of cycloalkanes. It then discusses nomenclature and several methods for preparing cycloalkanes, including from dihalogen compounds, calcium salts of carboxylic acids, and esters of dicarboxylic acids. The document also summarizes theories for explaining the stability and properties of cycloalkanes, such as Baeyer's strain theory, the Coulson-Moffitt modification, and the Sachse-Mohr theory. Finally, it outlines some common chemical reactions of cycloalkanes, including substitution, addition (ring-opening), and oxidation reactions.









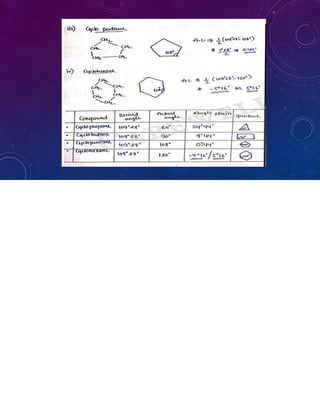
![Angle strain: It is angle difference between desire angle (109°28’)
and actual angle.
* Angle strain =1/2 [desire angle –actual angle]
=1/2[ 109°28’-actual angle]](https://image.slidesharecdn.com/pocppt-211022050947/85/Cycloalkanes-14-320.jpg)