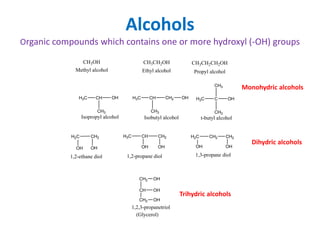
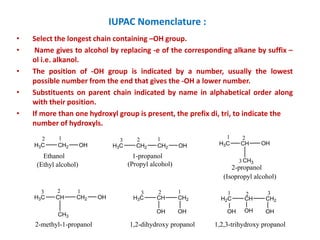
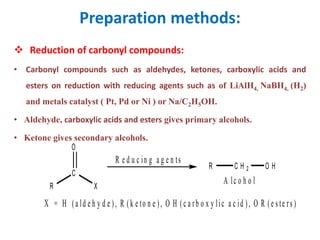
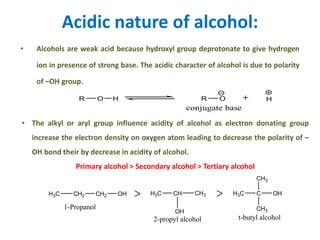
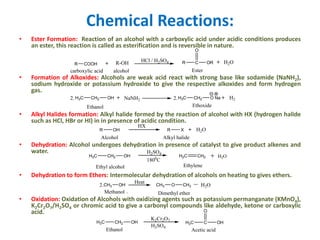
The document discusses alcohols, which are organic compounds containing hydroxyl (-OH) groups, detailing their nomenclature, preparation methods, acidic nature, chemical reactions, and physical properties. It explains the IUPAC naming conventions, various preparation techniques like reduction of carbonyl compounds, and chemical behaviors such as ester formation and dehydration. Additionally, it highlights the properties of alcohols, including boiling points, solubility, and their ability to form hydrogen bonds.