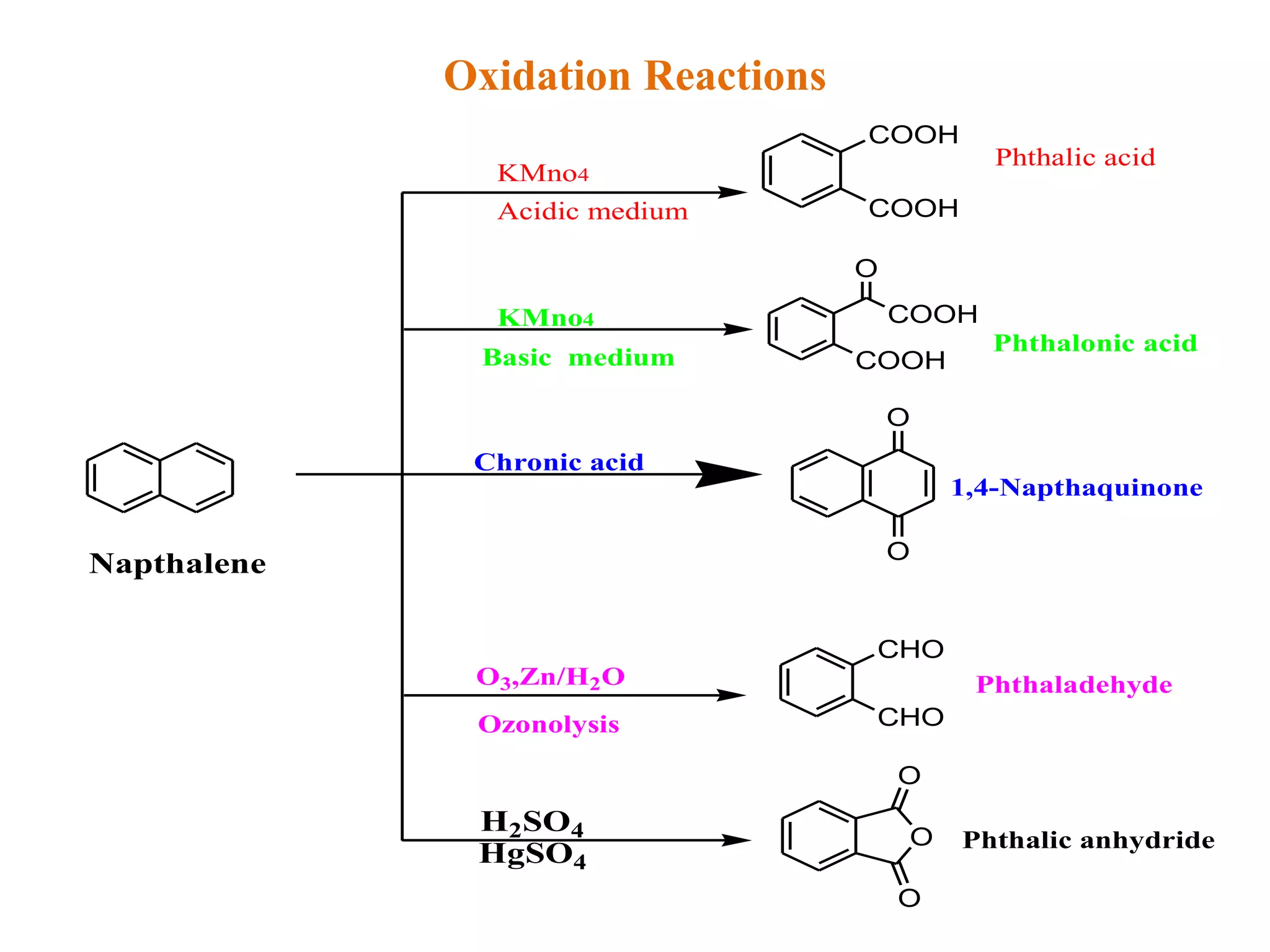
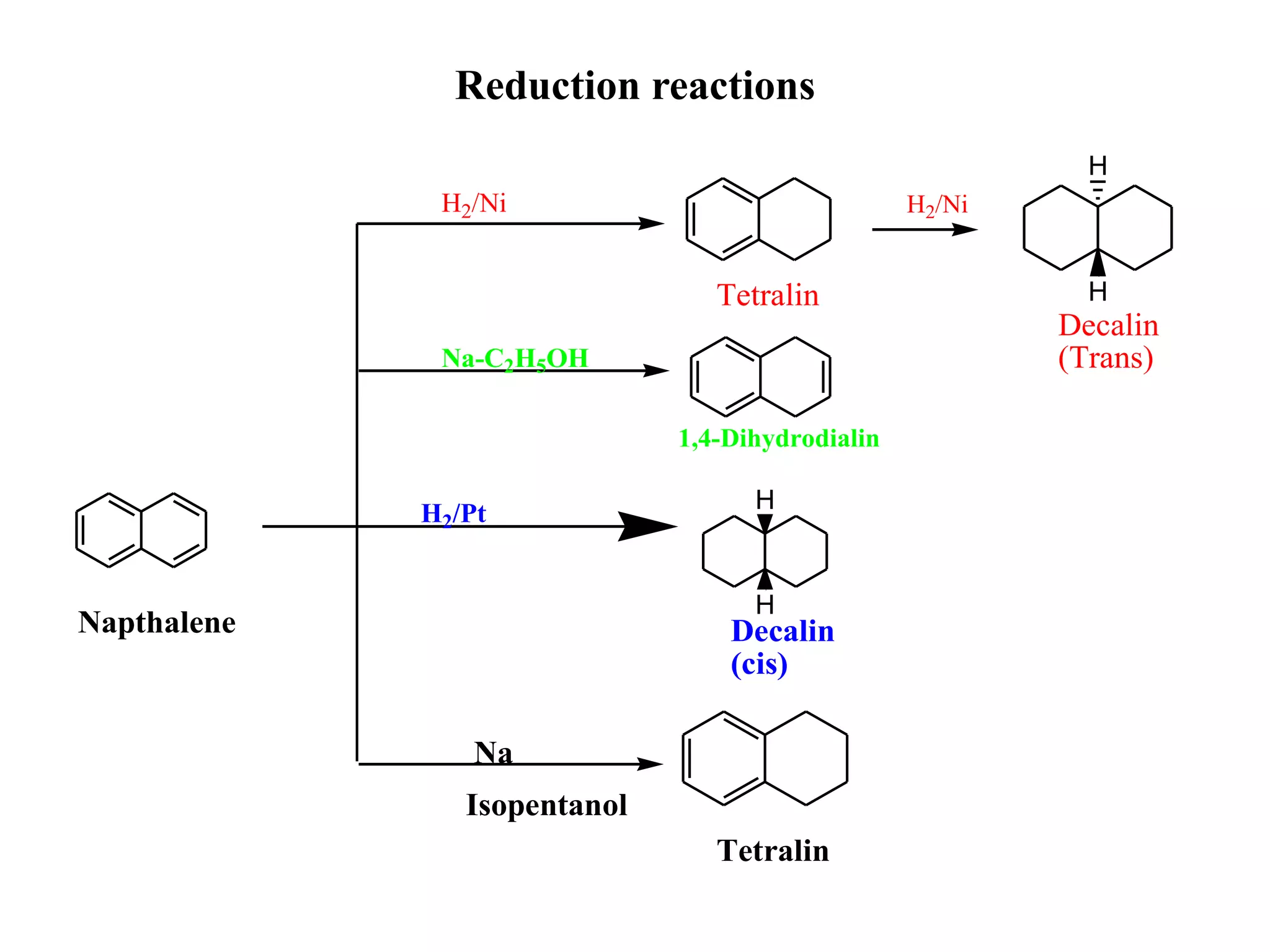
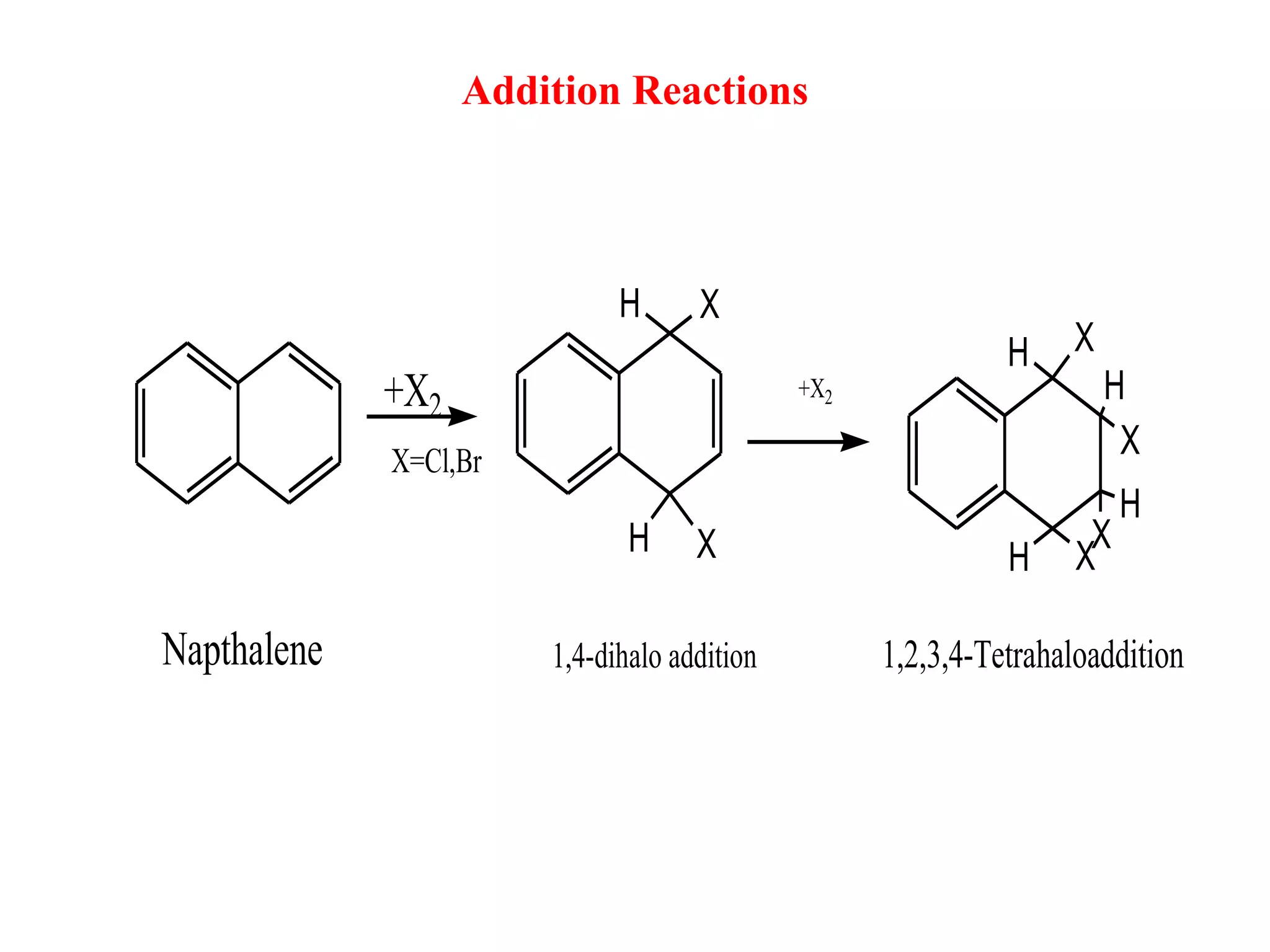
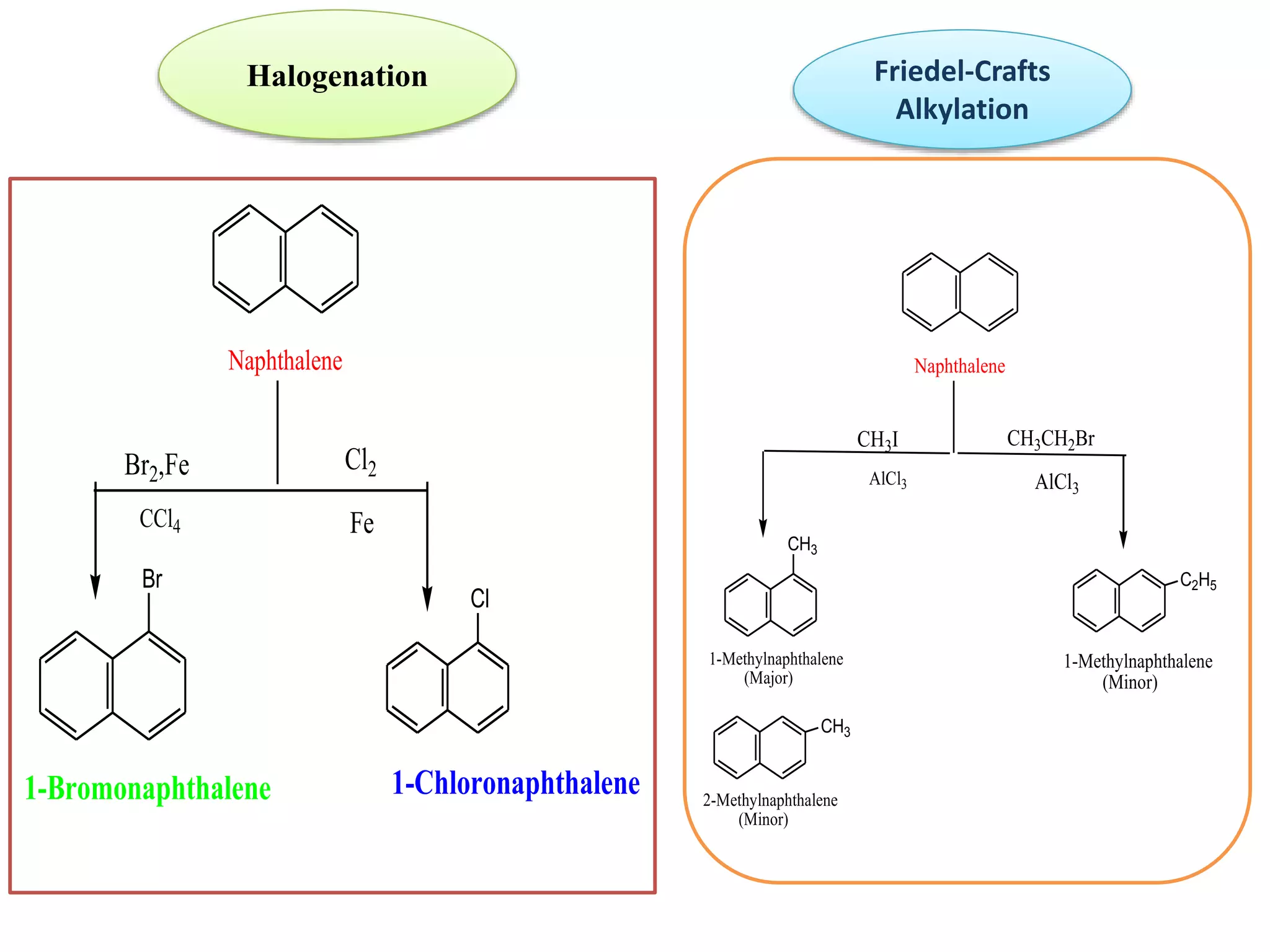
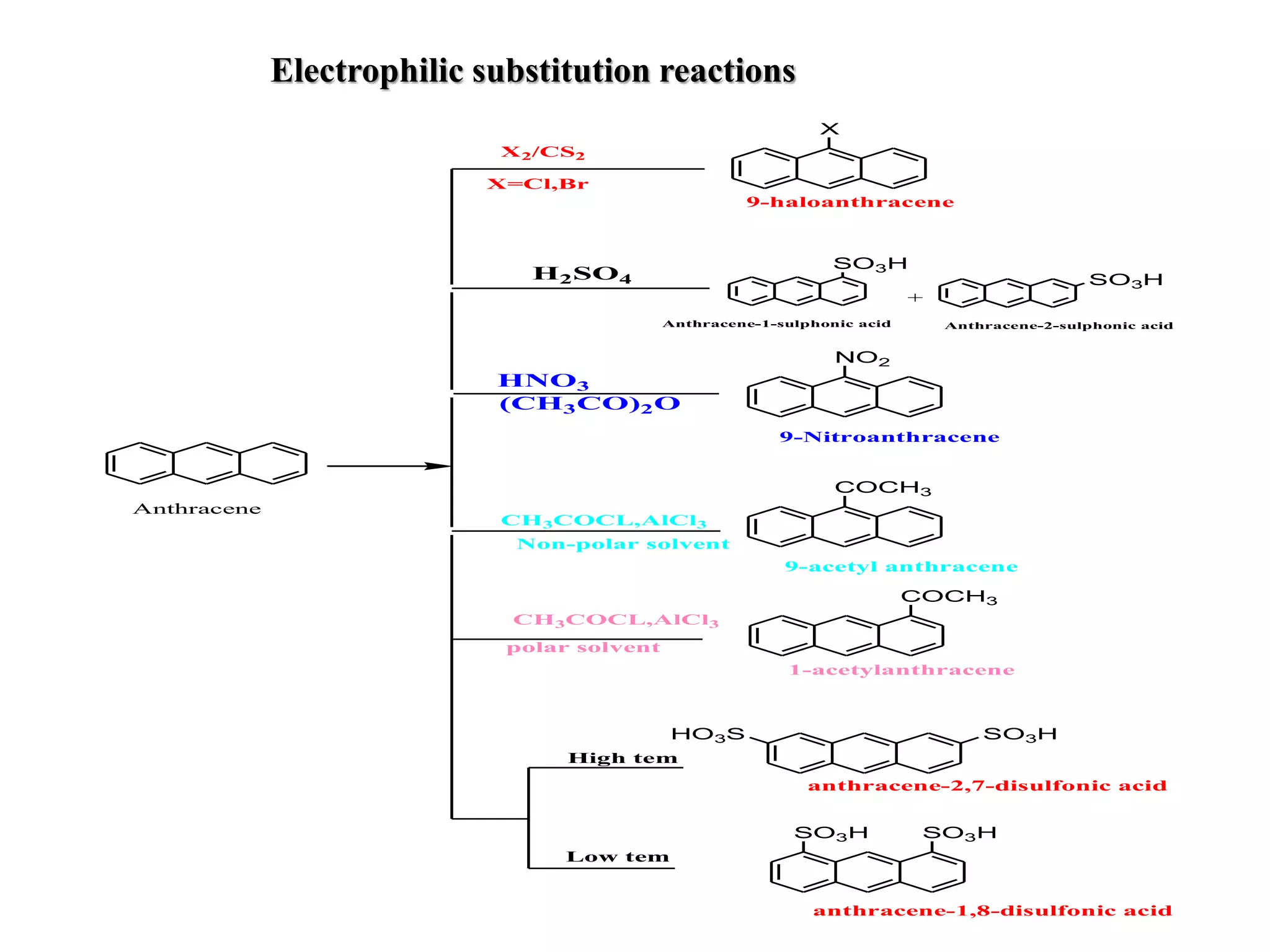
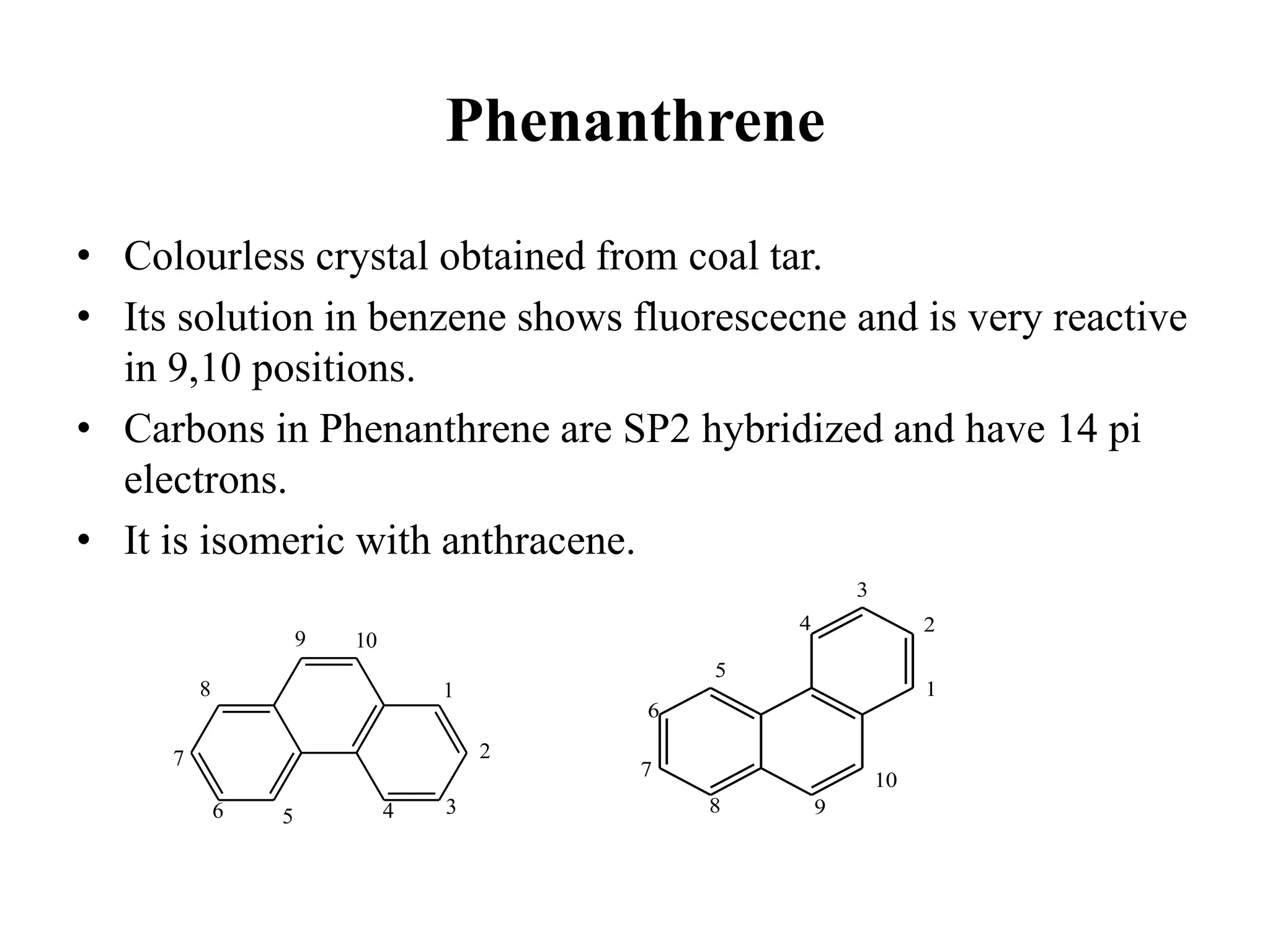
The document summarizes reactions of naphthalene, anthracene and phenanthrene. It discusses oxidation, reduction, addition and electrophilic substitution reactions of naphthalene. For anthracene, it describes its synthesis via Friedel-Crafts reactions and Diels-Alder reactions. Electrophilic substitution reactions of anthracene are also outlined. Phenanthrene synthesis is explained using Haworth and Pschorr syntheses.