This document discusses properties of pure substances and provides examples and explanations. It includes:
- Definitions of simple systems, homogeneous substances, and pure substances. Pure substances can exist in multiple phases like water as a solid, liquid, or vapor.
- The state postulate states the equilibrium state of a pure substance is determined by two intensive properties.
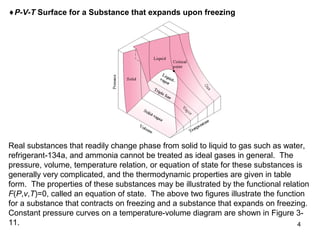
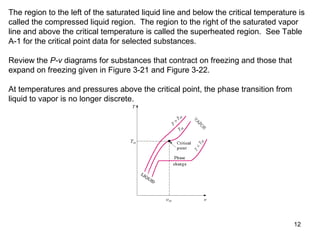
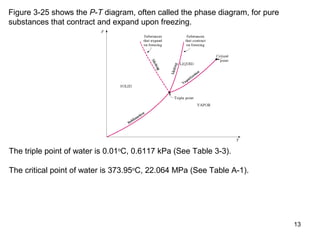
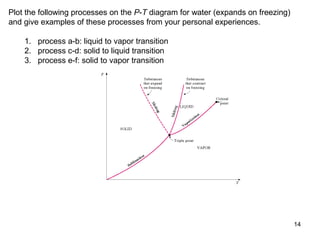
- Graphs of pressure-volume-temperature surfaces show relationships between solid, liquid, and gas phases for substances that contract or expand upon freezing.
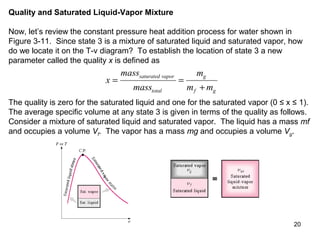
- Tables of thermodynamic properties like temperature, pressure, volume, energy and entropy for saturated water are presented to illustrate phase changes at constant pressure.





















![29
Example 2-1
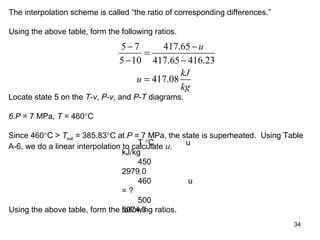
Find the internal energy of water at the given states for 7 MPa and plot the states on
T-v, P-v, and P-T diagrams.
10-4
10-3
10-2
10-1
100
101
102
103
103
0
100
200
300
400
500
600
700700
v [m
3
/kg]
T[C]
7000 kPa
Steam](https://image.slidesharecdn.com/chapter03-141217105701-conversion-gate01/85/Chapter03-pure-substance-29-320.jpg)
![30
10-4
10-3
10-2
10-1
100
101
102
102
100
101
102
103
104
105
v [m
3
/kg]
P[kPa]
374.1 C
285.9 C
Steam
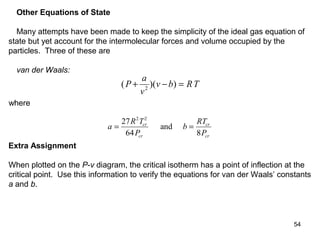
P
T °C
7
MPa
0.01 285.8 373.95
CPSteam
Triple
Point](https://image.slidesharecdn.com/chapter03-141217105701-conversion-gate01/85/Chapter03-pure-substance-30-320.jpg)


















![50
Tcr = 126.2 K, Pcr = 3.39 MPa R = 0.2968 kJ/kg-K
T
T
T
K
K
P
P
P
MPa
MPa
R
cr
R
cr
= = =
= = =
300
126 2
2 38
8 0
339
2 36
.
.
.
.
.
Since T > 2Tcr and P < 10Pcr, we use the ideal gas equation of state
Pv RT
v
RT
P
kJ
kg K
K
MPa
m MPa
kJ
m
kg
=
= =
−
=
0 2968 300
8 0 10
0 01113
3
3
3
. ( )
.
.
Nitrogen is clearly an ideal gas at this state.
If the system pressure is low enough and the temperature high enough (P and T are
compared to the critical values), gases will behave as ideal gases. Consider the T-v
diagram for water. The figure below shows the percentage of error for the volume ([|
vtable – videal|/vtable]x100) for assuming water (superheated steam) to be an ideal gas.](https://image.slidesharecdn.com/chapter03-141217105701-conversion-gate01/85/Chapter03-pure-substance-50-320.jpg)




![56
Example 2-8
Compare the results from the ideal gas equation, the Beattie-Bridgeman equation,
and the EES software for nitrogen at 1000 kPa. The following is an EES solution to
that problem.
10-3
10-2
10-1
10-1
70
80
90
100
110
120
130
140
150
160
v [m
3
/kg]
T[K]
1000 kPa
Nitrogen, T vs v for P=1000 kPa
EES Table ValueEES Table Value
Beattie-BridgemanBeattie-Bridgeman
Ideal GasIdeal Gas
Notice that the results from the Beattie-Bridgeman equation compare well with the
actual nitrogen data provided by EES in the gaseous or superheated region.
However, neither the Beattie-Bridgeman equation nor the ideal gas equation provides
adequate results in the two-phase region, where the gas (ideal or otherwise)
assumption fails.](https://image.slidesharecdn.com/chapter03-141217105701-conversion-gate01/85/Chapter03-pure-substance-56-320.jpg)