The document discusses several key concepts in pharmacophore modeling:
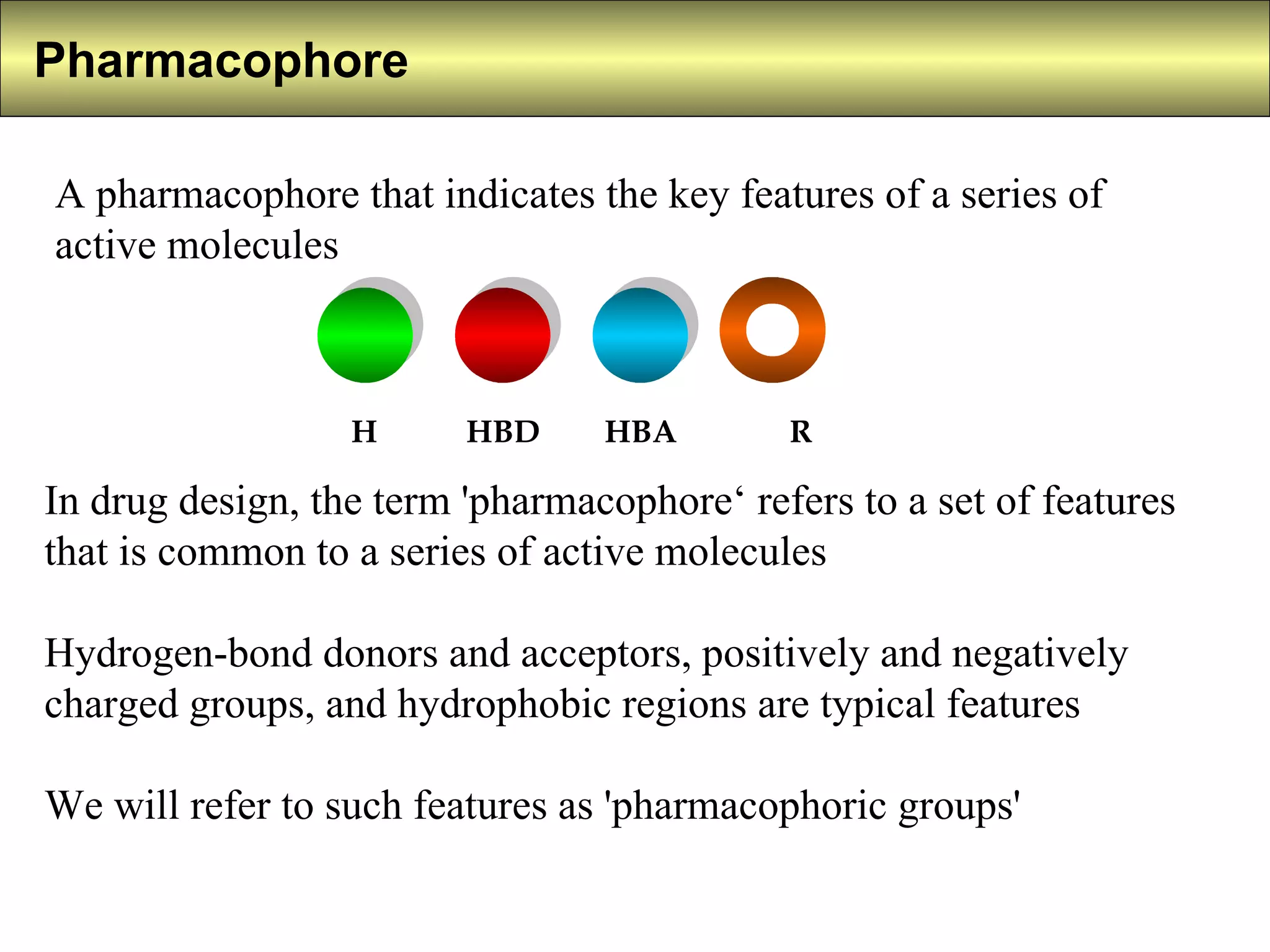
1) A pharmacophore defines the important chemical features shared among active molecules, such as hydrogen bond donors/acceptors and hydrophobic regions.
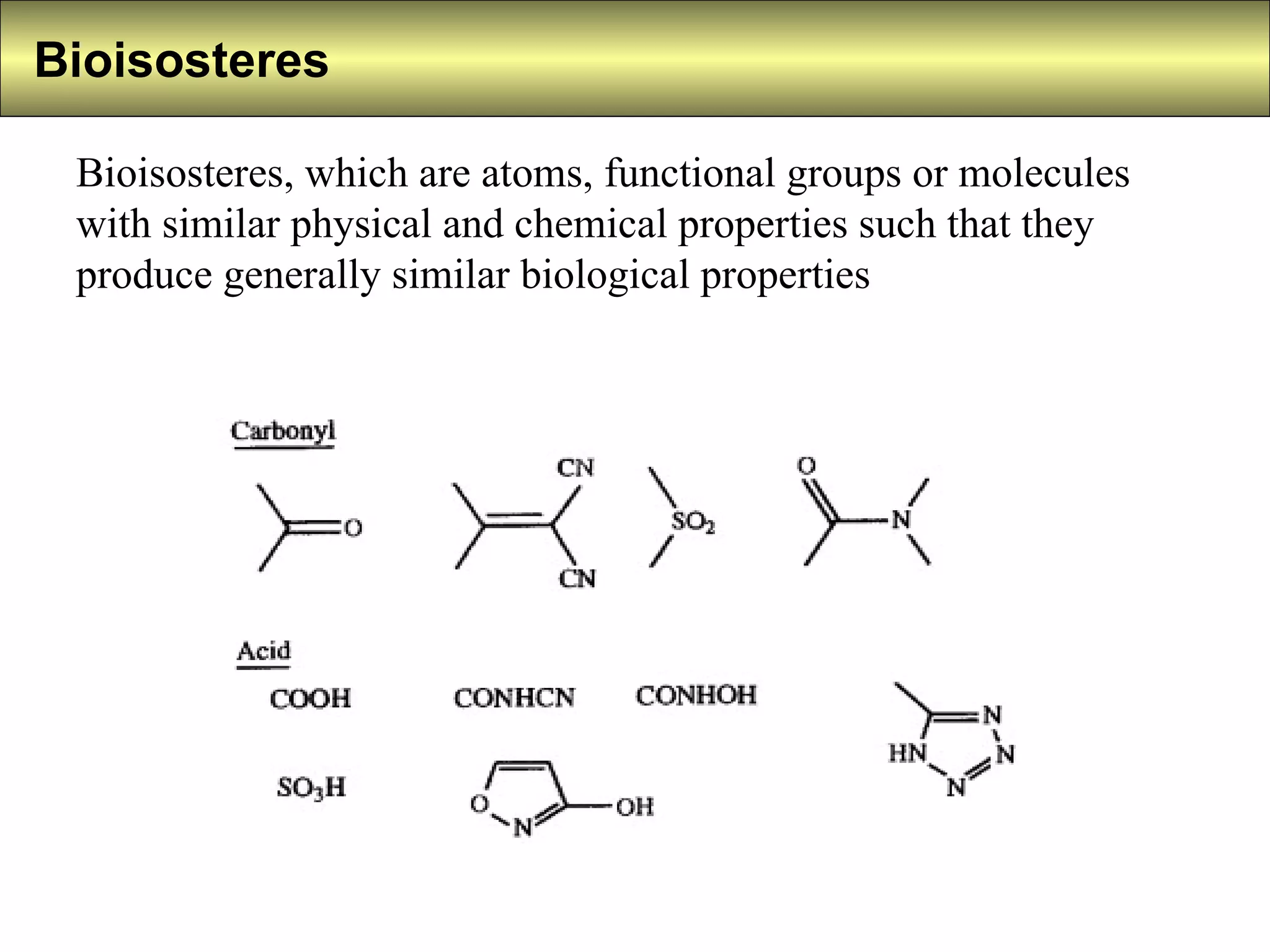
2) Bioisosteres are atoms or groups with similar physical/chemical properties that produce similar biological effects.
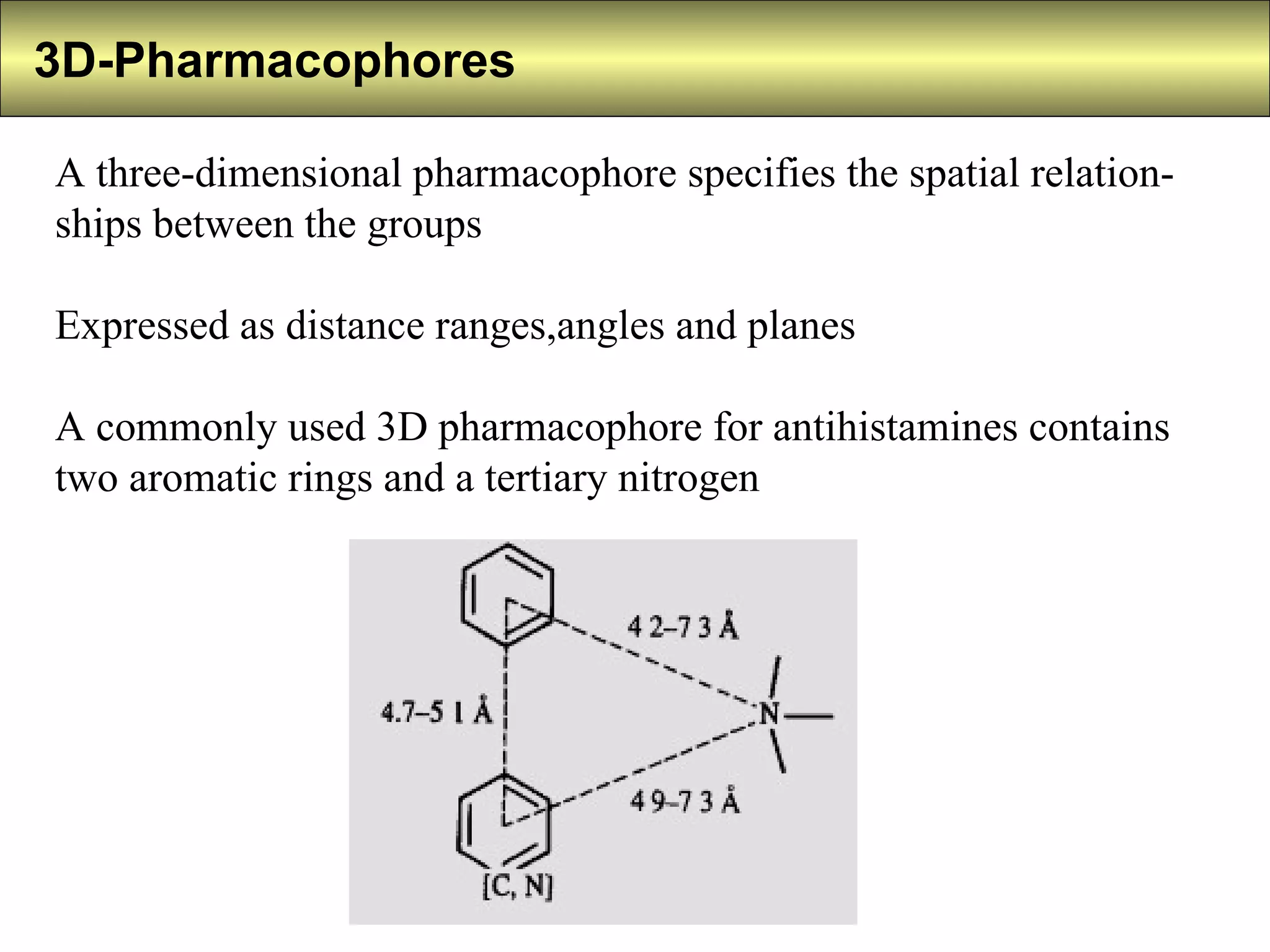
3) 3D pharmacophores specify the spatial relationships between features as distance ranges and angles.
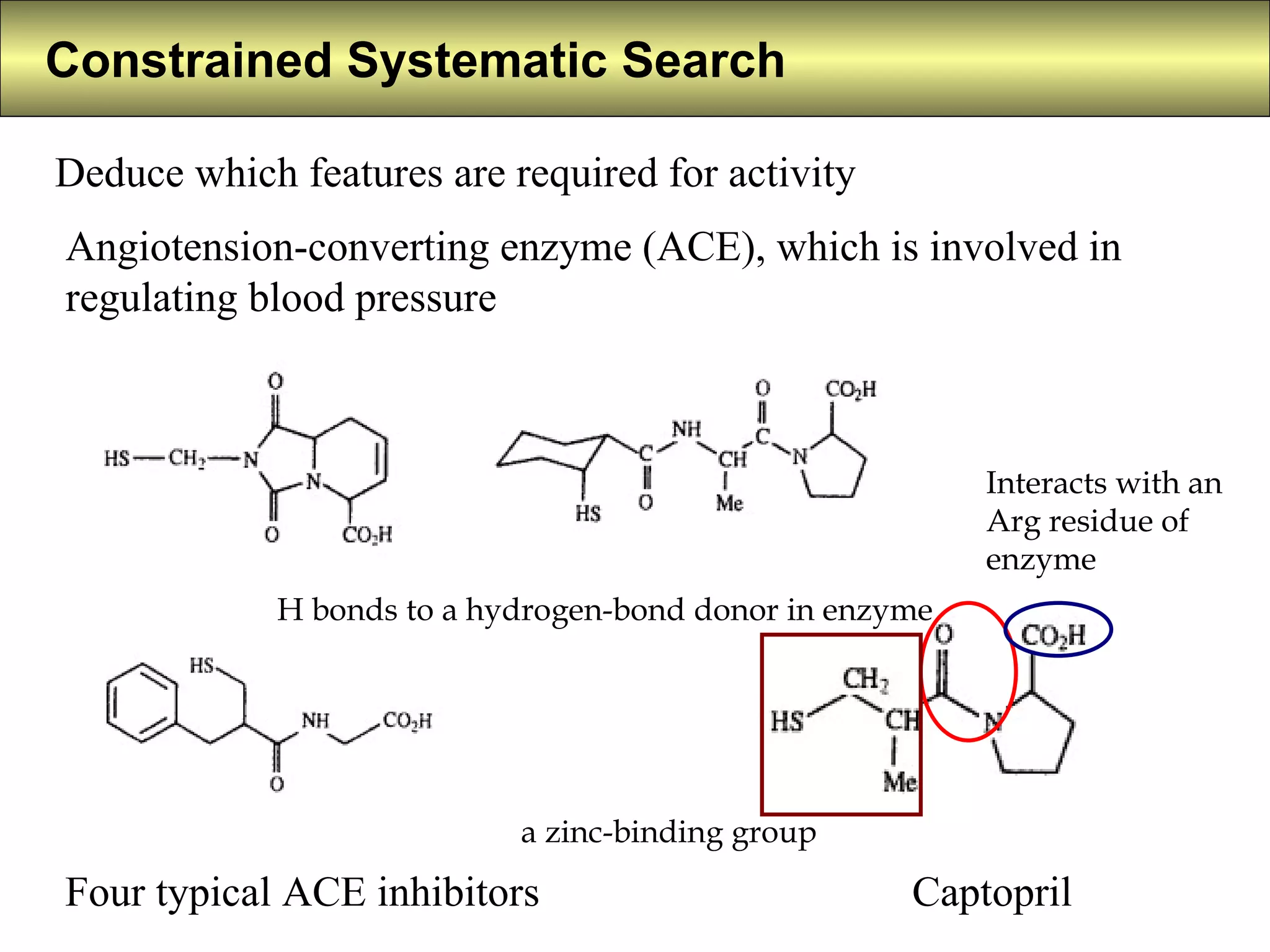
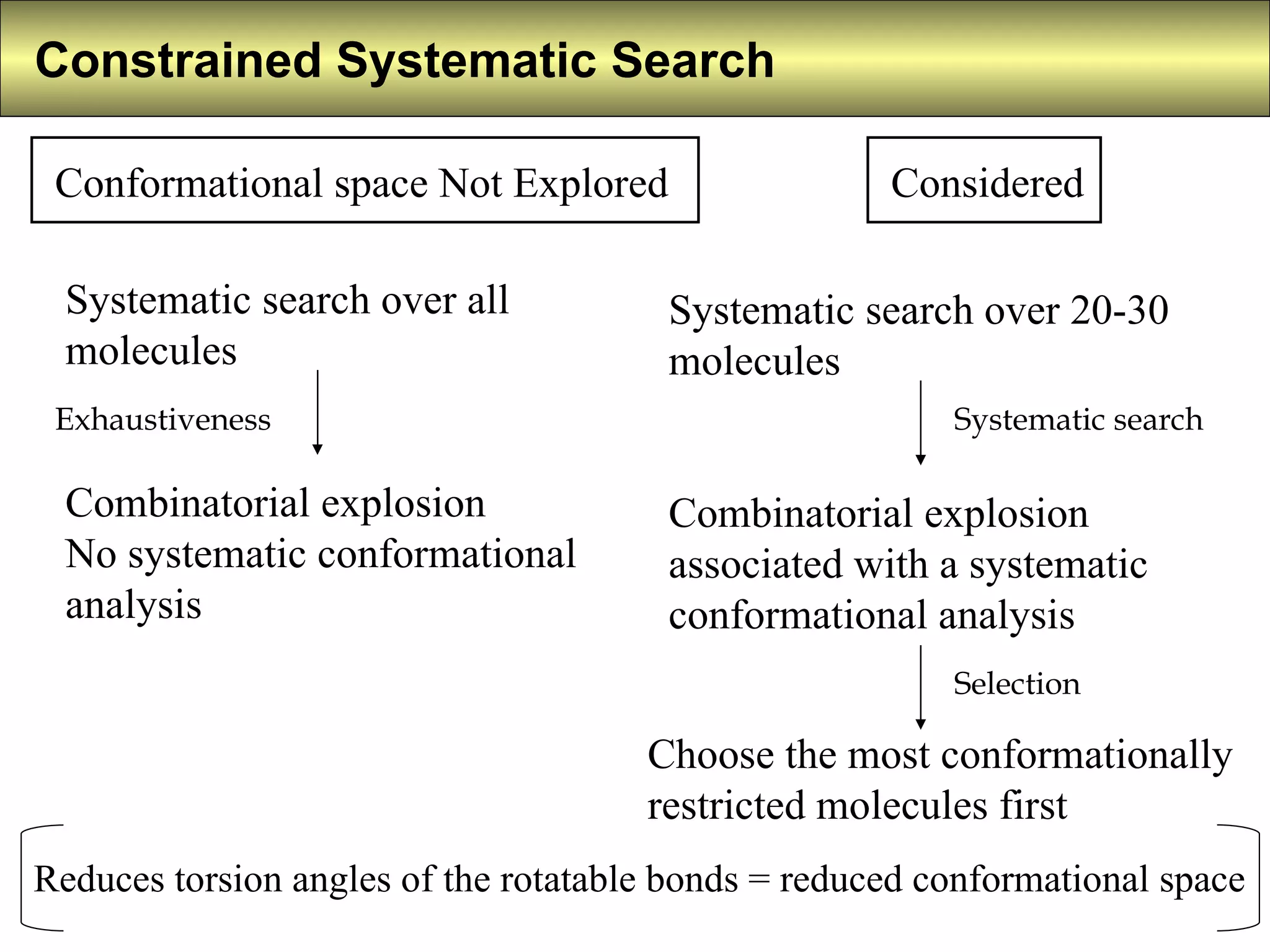
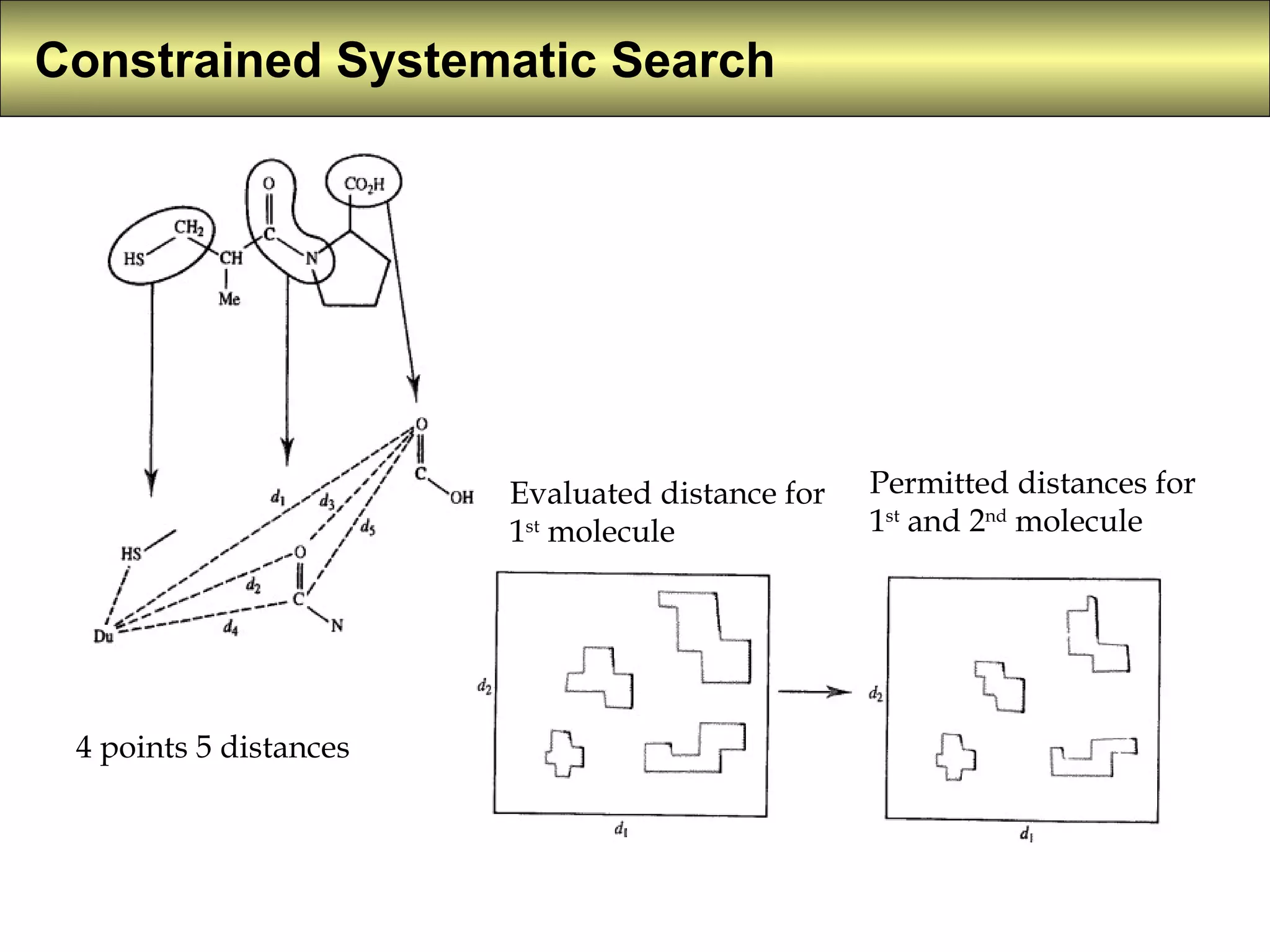
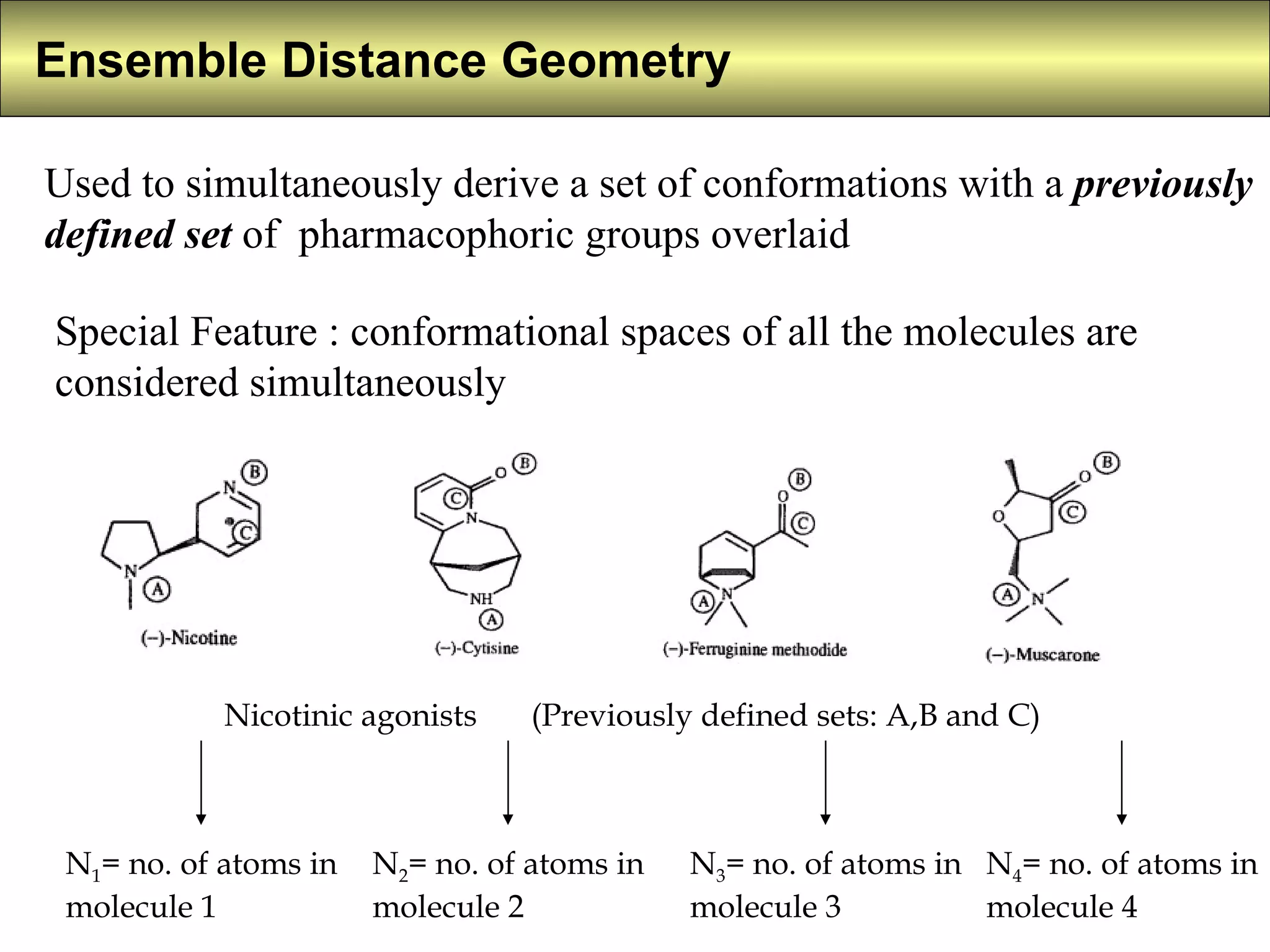
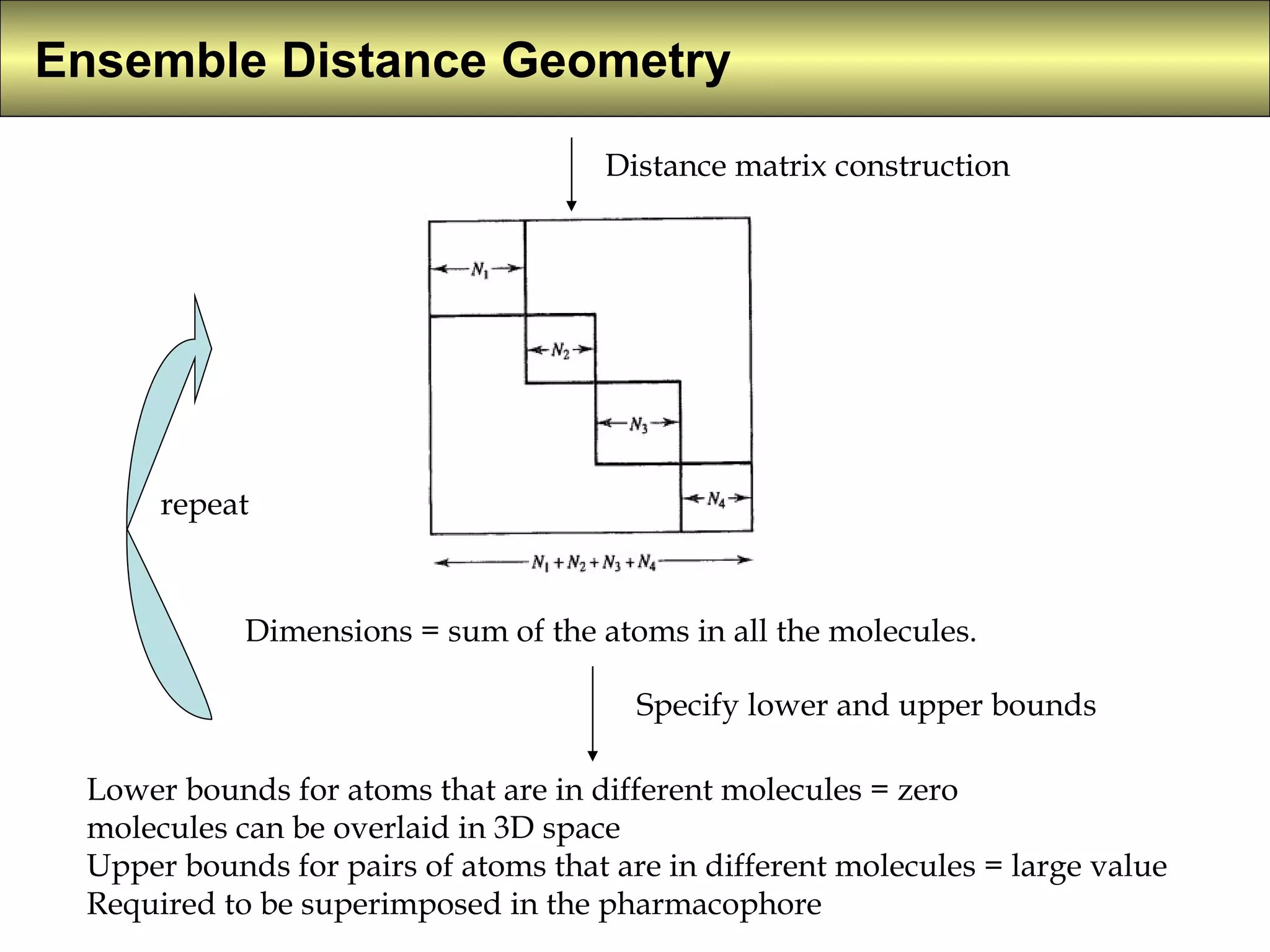
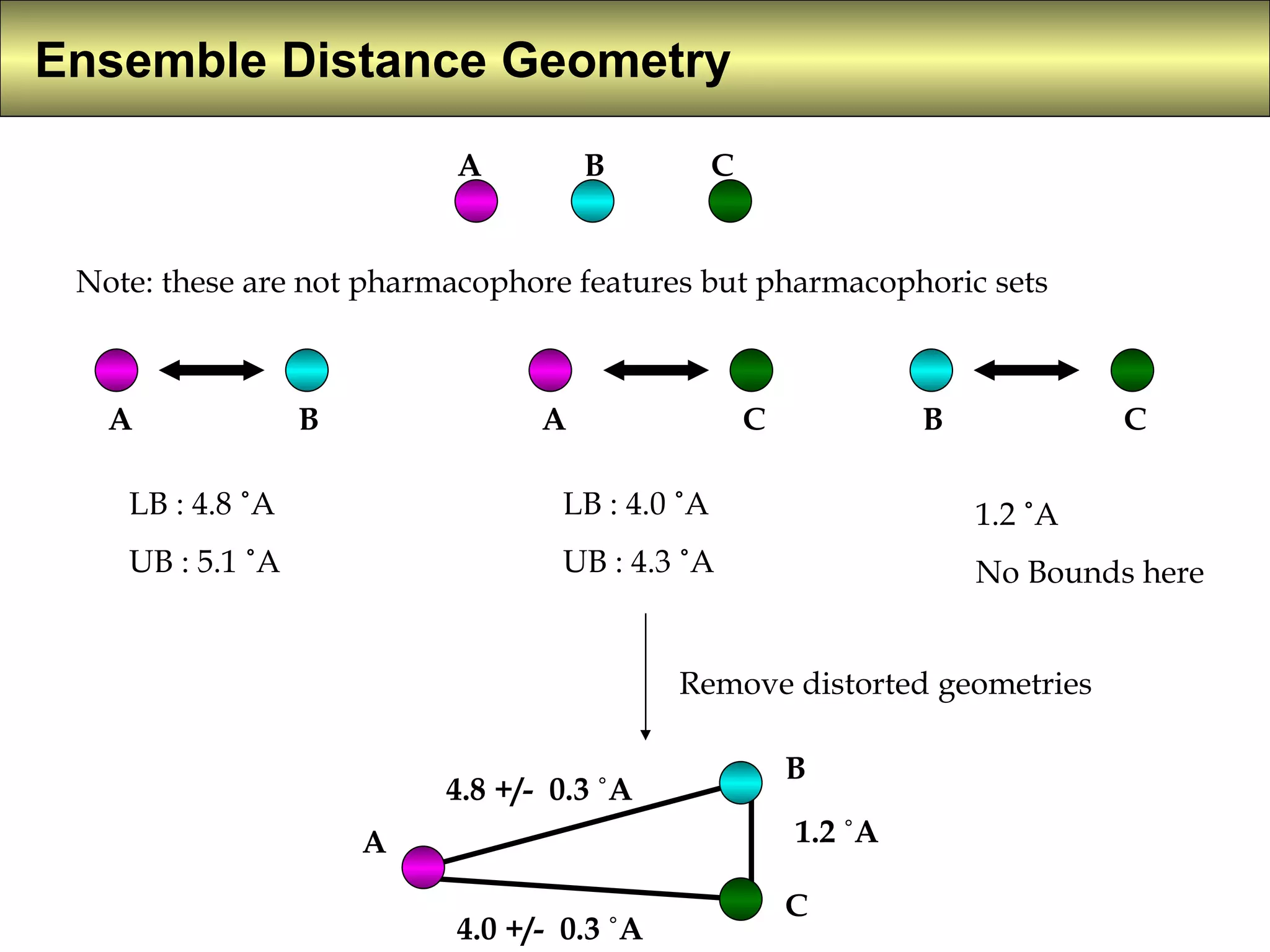
4) Constrained systematic searching and ensemble distance geometry are used to identify pharmacophores from a set of molecules while considering multiple conformations.
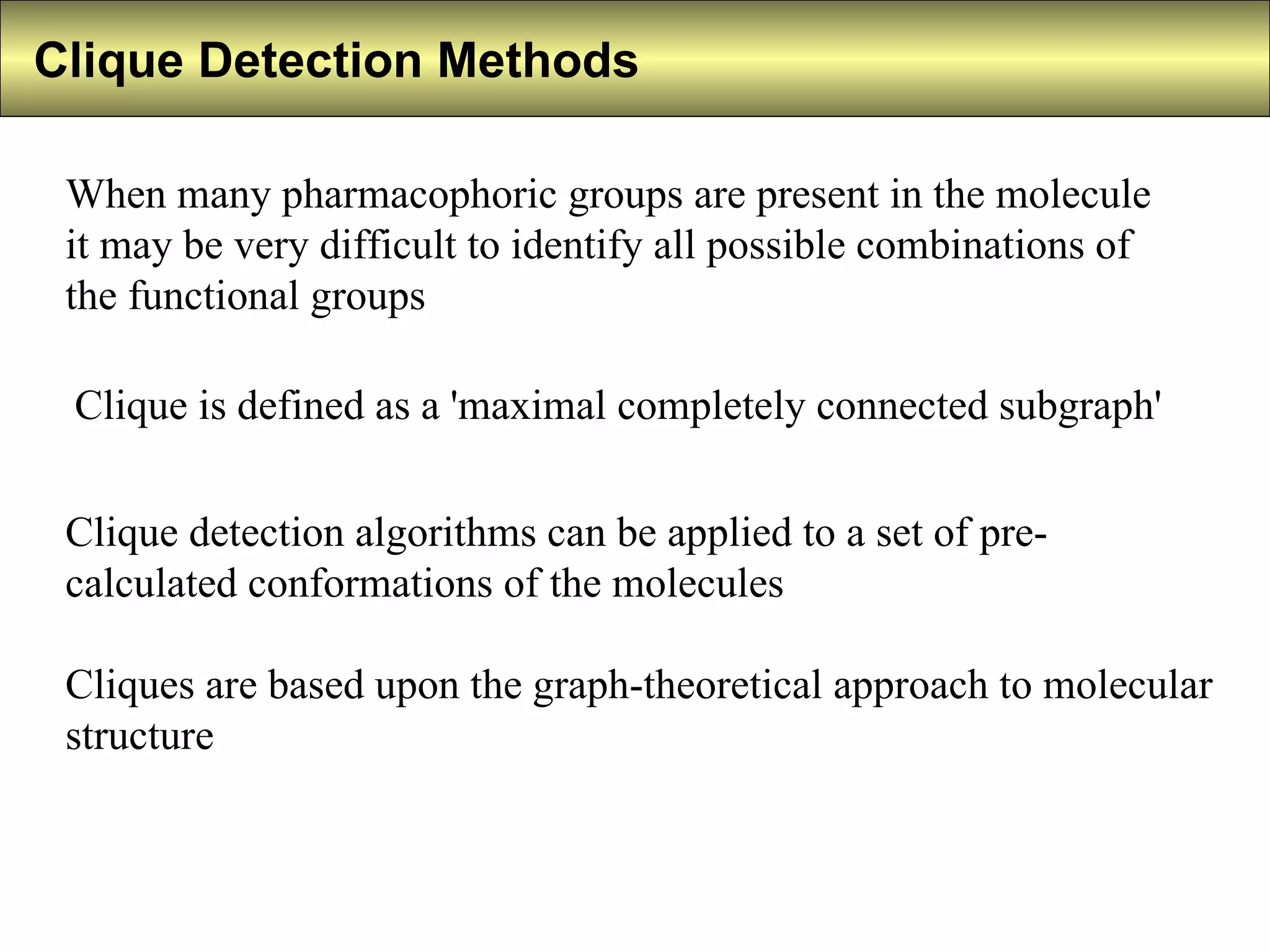
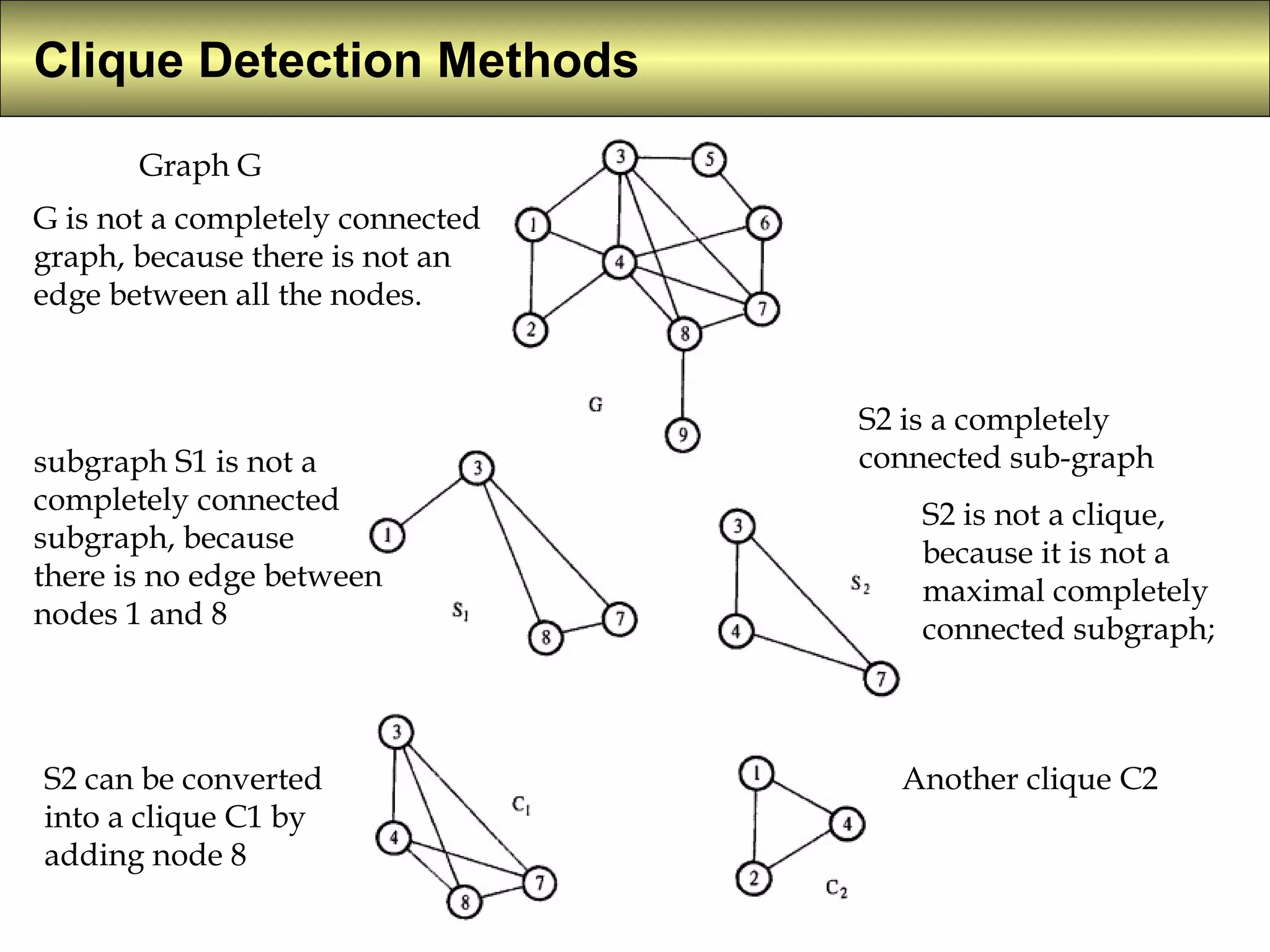
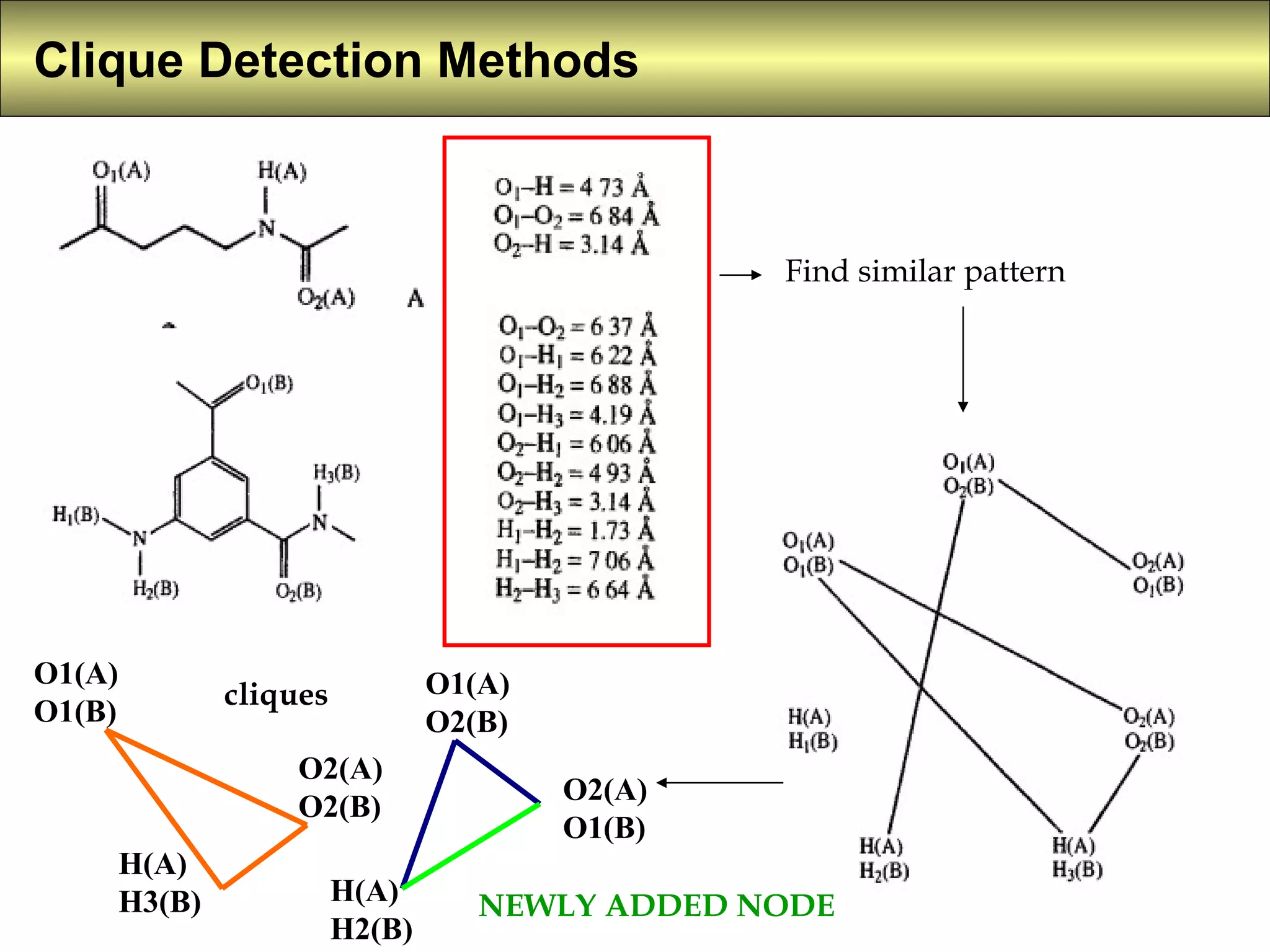
5) Clique detection identifies all possible combinations of pharmacophoric groups in molecules by finding "maximal completely connected subgraphs".
![S.Prasanth Kumar, Bioinformatician Drug Design Pharmacophore Identification S.Prasanth Kumar, Bioinformatician S.Prasanth Kumar Dept. of Bioinformatics Applied Botany Centre (ABC) Gujarat University, Ahmedabad, INDIA www.facebook.com/Prasanth Sivakumar FOLLOW ME ON ACCESS MY RESOURCES IN SLIDESHARE prasanthperceptron CONTACT ME [email_address]](https://image.slidesharecdn.com/pharmacophoreidentification-110324014000-phpapp01/75/Pharmacophore-identification-1-2048.jpg)