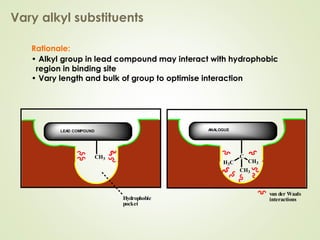
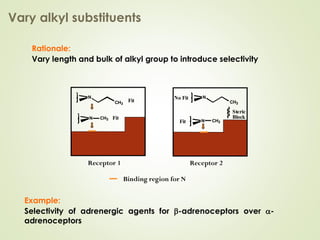
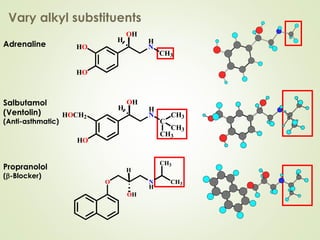
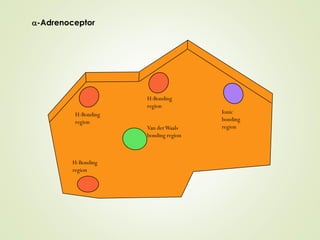
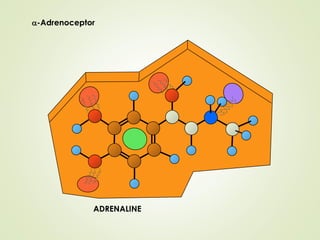
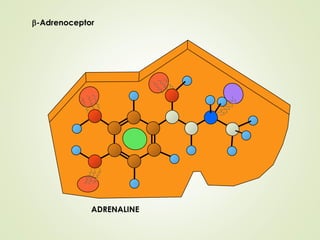
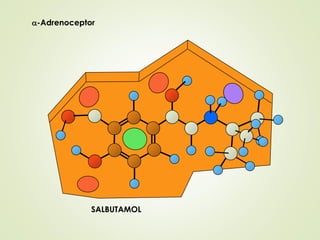
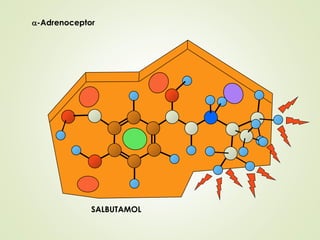
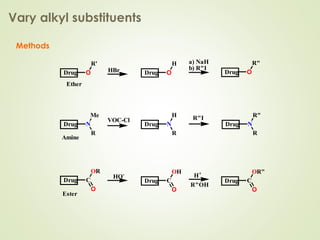
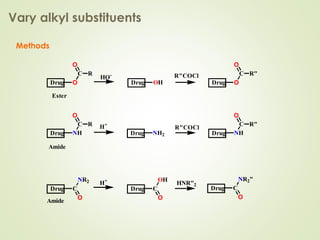
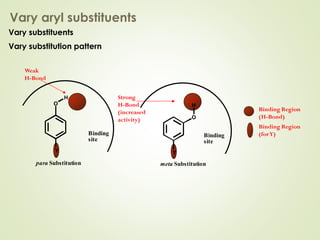
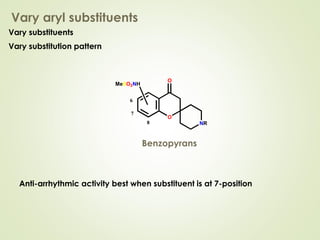
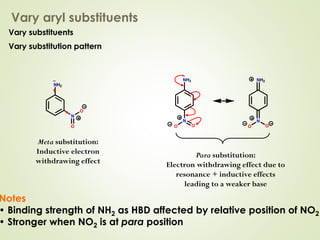
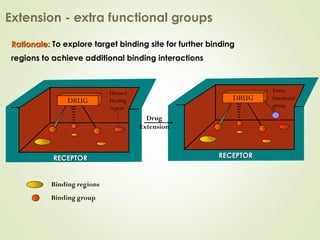
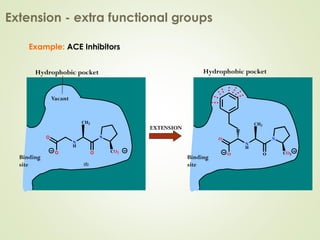
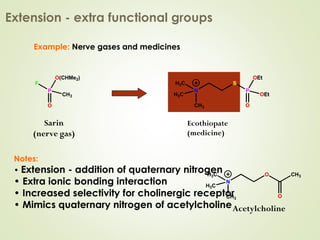
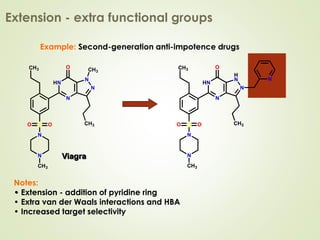
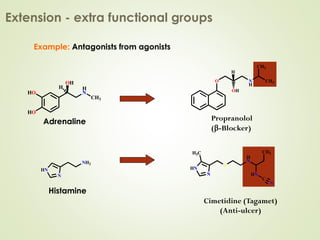
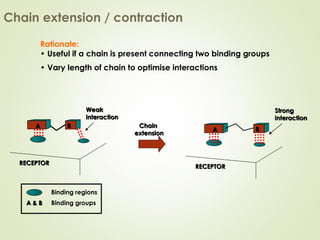
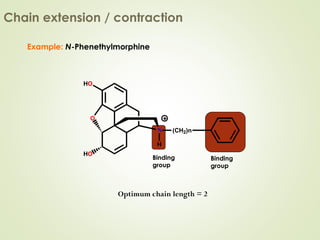
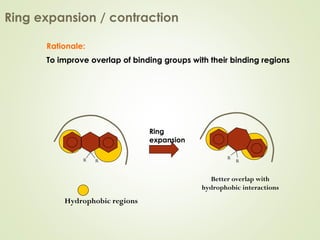
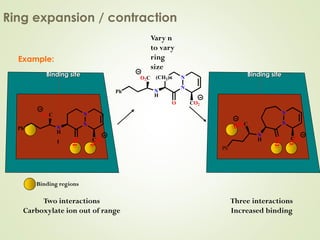
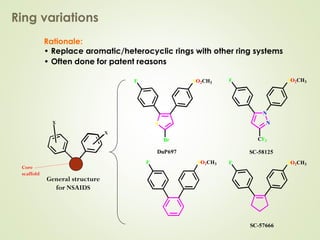
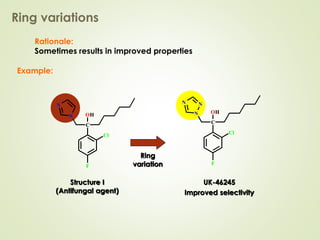
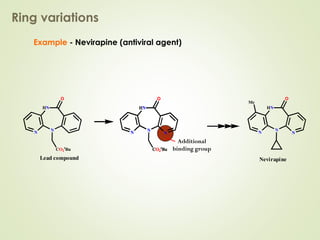
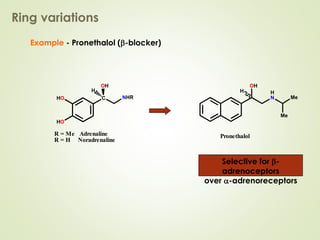
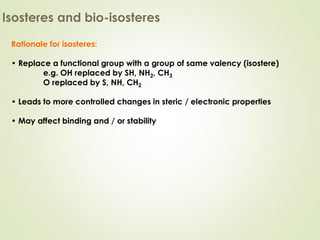
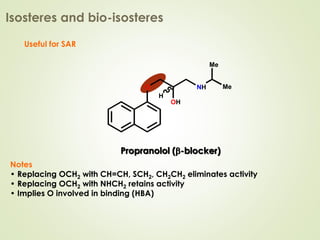
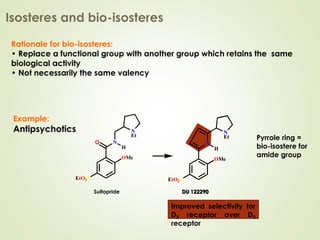
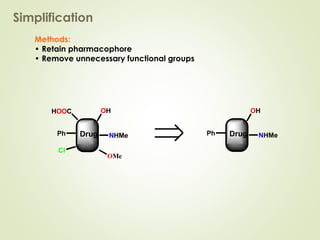
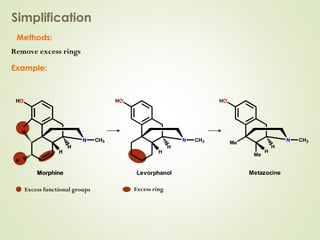
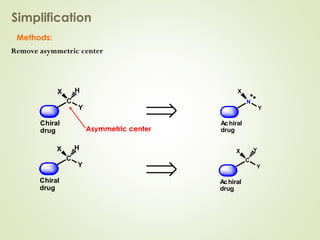
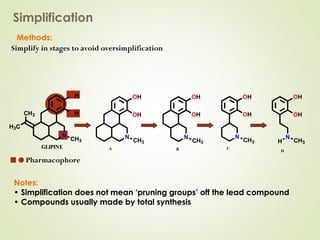
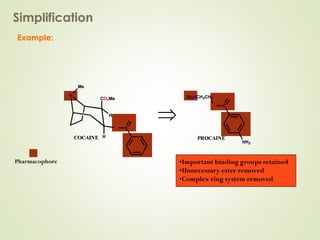
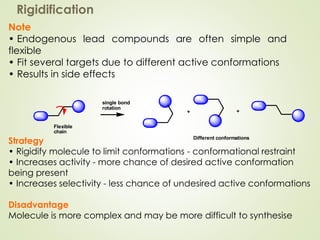
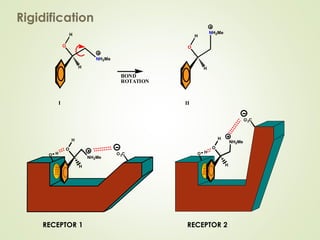
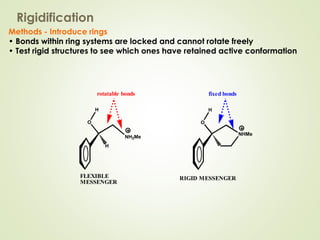
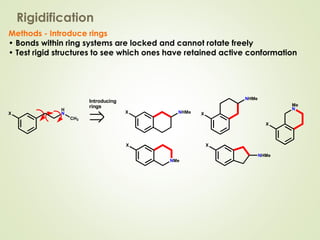
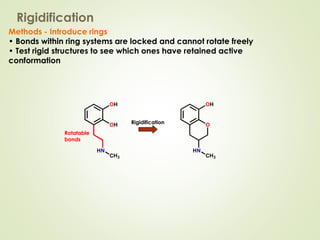
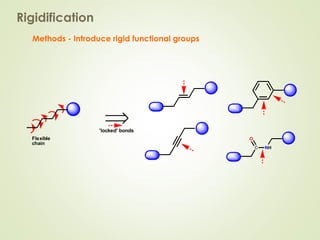
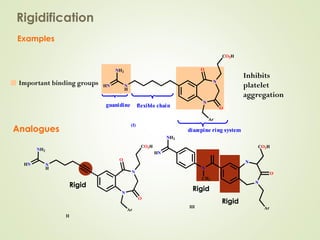
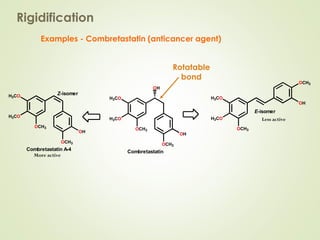
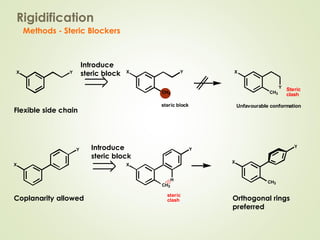
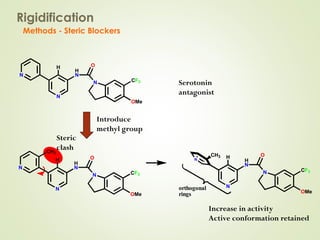
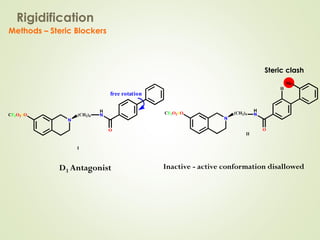
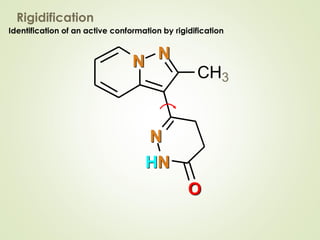
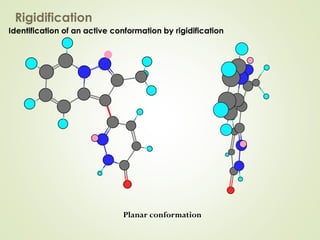
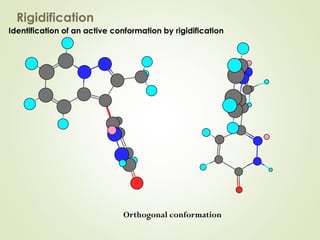
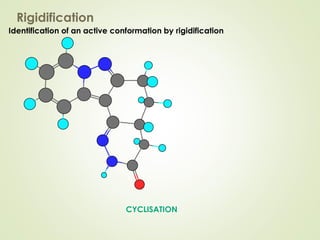
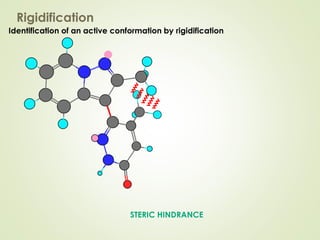
The document discusses strategies for drug design such as varying substituents on lead compounds to optimize binding interactions and selectivity. Key strategies include varying alkyl and aryl substituents, extending functional groups, and modifying ring systems through expansions, contractions, or isosteric replacements. Other approaches involve simplifying molecule structures while retaining essential pharmacophores or rigidifying flexible structures to limit undesirable conformations. The goal is to systematically modify lead compounds to improve desired target interactions and properties like potency, selectivity, and toxicity profile.