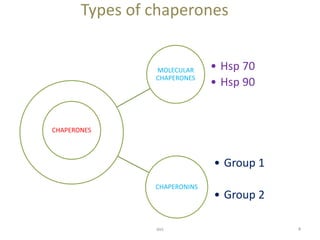
This document discusses different types of chaperone proteins. It begins by explaining that chaperones assist other proteins in folding correctly by interacting with unfolded or misfolded proteins. The main types discussed are molecular chaperones like Hsp70 and Hsp90, and chaperonins. Hsp70 binds to hydrophobic regions of unfolded proteins to prevent aggregation, while Hsp90 helps activate client proteins. Chaperonins form folding chambers and there are two groups - group 1 found in prokaryotes and organelles, and group 2 in eukaryotes and archaea. Specific examples of chaperone homologs in different organisms are also provided.