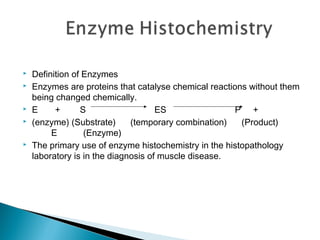
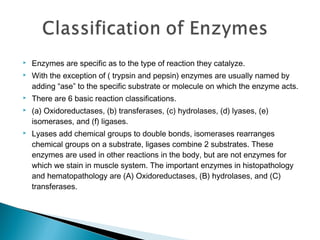
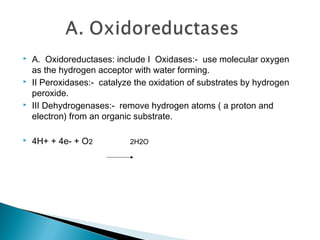
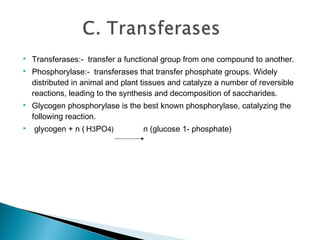
Enzymes are proteins that catalyze chemical reactions without being changed chemically. They act as catalysts by lowering the activation energy of reactions and increasing their rate. Enzymes are usually named by adding "ase" to the substrate they act on and can be classified into six groups including oxidoreductases, transferases and hydrolases. Enzyme histochemistry is used in muscle disease diagnosis by demonstrating enzyme activity and localization in tissue sections.